Staphylococcal pyomyositis is a severe invasive soft tissue infection with high mortality rate that is increasingly being recognized even in temperate climates. In most cases predisposing factors are identified that include either source of skin penetration or/and impaired host immunocompetence. A case of primary, community-acquired pyomyositis of the left iliopsoas muscle in a 59-year-old immunecompetent woman, which was complicated with septic pulmonary emboli within 24h after hospital admission, is presented. The patient was subjected to abscess drainage under computed tomography guidance. Both pus aspiration and blood cultures revealed methicillin-susceptible Staphylococcus aureus. Given the absolute absence of predisposing factors and a remote history of staphylococcal osteomyelitis in the same anatomical region 53 years ago, reactivation of a staphylococcal soft tissue infection was postulated. Systematic review of the literature revealed a few interesting cases of reactivated staphylococcal infection after decades of latency, although the exact pathophysiological mechanisms still need to be elucidated.
Primary pyomyositis is an acute bacterial infection characterized by suppuration within large skeletal muscles manifesting as single or multiple abscesses.1–3Staphylococcus aureus is the leading causative agent (70–90% of all cases). This invasive soft tissue infection was traditionally encountered in tropical countries, where concomitant parasitic infections, nutritional deficiencies and repetitive lower extremity trauma due to barefooted walking may have contributed to its pathogenesis.1 In temperate climates, primary pyomyositis had been considered rare, with only 98 cases being reported in North America from 1971 to 1992.1,2 Currently, many cases are being reported worldwide with increased incidence and high mortality of around 10%, which may reach 20–60% in short terms with concomitant sepsis.4,5 Predisposing factors are almost always identified, and include either skin penetration (for example intravenous drug use, intramuscular injections, external wounds or trauma, underlying skin disease) or impaired host immunocompetence like infection with human immunodeficiency virus, diabetes mellitus, malignancy, connective tissue diseases, cirrhosis, and immunosuppressive therapy.2
Apart from host predisposing factors, recent advances in microbiology have linked invasive soft tissue staphylococcal infections with the production of the Panton–Valentine leukocidin (PVL) toxin.6,7 PVL is a member of the synergohymenotropic family of exotoxins that destroy leukocytes by creating pores in the cell membrane and induce tissue necrosis at the site of infection.6 This toxin is believed to be a potent factor of virulence that contributes significantly to increased morbidity and mortality from both methicillin-sensitive (MSSA) and methicillin-resistant S. aureus (MRSA) infections.7 Further studies have also concluded that production of PVL is associated with higher rates of recurrent invasive staphylococcal infections irrespective of methicillin susceptibility.7
In this study we report an interesting case of primary, community-acquired pyomyositis in a Greek immunecompetent woman, which was rapidly complicated with septic pulmonary emboli. Given the absolute absence of predisposing factors and a remote history of staphylococcal osteomyelitis in the same anatomical region 53 years ago, reactivation of a latent staphylococcal soft tissue infection was postulated. Systematic review of the literature revealed a few interesting cases of reactivated staphylococcal infections,8–14 although the distinct pathophysiological mechanisms still need to be elucidated.
Case reportA 59-year-old woman was referred to our hospital because of high temperature, orthostatic hypotension and left thigh pain. The patient was in good condition until 15 days earlier, when back pain reflecting to the left hip and thigh developed. The pain worsened gradually and two days earlier fever developed accompanied with chills, sweats, and extreme fatigue. The patient was temporally relieved from symptoms after receiving antipyretic agents. The next day temperature rose to 39.5°C, and the patient presented unbearable thigh pain. She was finally referred to hospital for further investigation.
The patient was a mother and was working as administrative employee in another hospital. She was not under medication for any illness. She mentioned a surgical procedure to the left ilium due to staphylococcal osteomyelitis 53 years ago, at the age of six. The patient reported no concomitant diseases or skin infection, no recent trauma, bites or intramuscular injections. The patient was a smoker and did not exercise strenuously.
On admission, the initial evaluation of the vital signs revealed hypotension with systolic pressure of 70mmHg while lying down, pulse of 90 beats per minute, normal temperature, and respiratory rate of 15 breaths per minute with oxygen saturation of 96%. Chest X-ray and electrocardiogram were normal. Clinical examination revealed no thigh sensitivity, but the patient reported pain to the left hip and thigh that was exacerbated when performing movement. The remaining examination was normal. Initial laboratory investigation showed mildly elevated white blood cell count with neutrophilic predominance, elevated inflammatory markers (erythrocyte sedimentation rate, C-reactive protein, procalcitonin), and only mildly elevated liver enzymes and creatinine kinase.
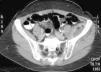
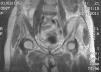
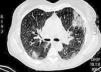
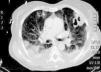
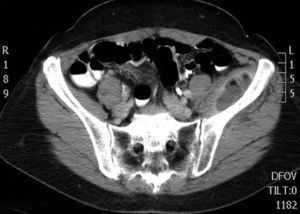
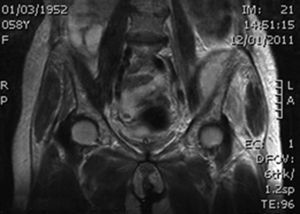
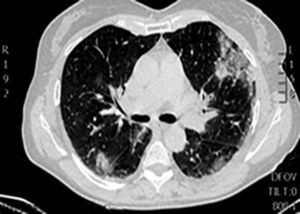
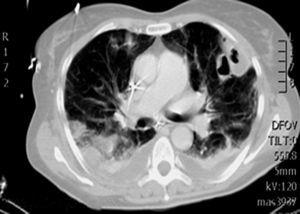
During the first hours of hospitalization, the patient was found febrile, and blood cultures were obtained. Antipyretics and empirical antibiotics against both Gram-positive and Gram-negative microorganisms were administered. Whole body imaging with computed tomography (CT) revealed an abscess within the left iliopsoas muscle (Fig. 1), and few smaller ones in the gluteus muscle, findings that were confirmed with magnetic resonance imaging (MRI) (Fig. 2). No adjacent bone changes were detected. Abscess was drained under CT guidance, and pus aspiration was sent to culture. Hours later the patient complained for new-onset sudden bilateral pleuritic pain with accompanied difficulty in breathing. On auscultation, abnormal breath sounds with crackles and diffuse rhonchi rapidly developed, while hypoxia was observed in oxymetry. New X-ray showed bilateral opacities, and new CT of the thorax revealed the presence of nodular infiltrates in both pulmonary fields (Fig. 3). Differential diagnosis included septic pulmonary emboli or acute respiratory distress syndrome (ARDS). Investigation for right-sided endocarditis with trans-eosophageal echocardiogram, as well as for thrombosis in the lower extremities proved negative. Despite all actions, the patient's health was deteriorating and he was brought to the Intensive Care Unit due to acute respiratory failure requiring mechanical ventilation. On the fifth day, a methicillin-susceptible strain of S. aureus was isolated from blood and pus, while cultures of skin and nares were negative. The patient improved under anti-staphylococcal chemotherapy with linezolid 600mg intravenously, and four days later, she was weaned from the ventilator. Repeated CT within 15 days revealed reduction of the iliopsoas abscess and of the cavitation of the nodular lung infiltrates (Fig. 4), confirming the diagnosis of septic pulmonary emboli due to staphylococcal pyomyositis. She continued on intravenous antibiotics for four weeks followed by four weeks of oral treatment. Repeated CT within three months after the end of treatment showed complete disappearance of abscesses.
Primary pyomyositis is an intriguing disease, as skeletal muscles are intrinsically resistant to bacterial infections.2 However, under certain circumstances, S. aureus can practically invade all muscle groups by being seeded from transient bacteremia, and without an apparent spread from contiguous structures.3 On the other hand, when abscesses extend into muscles from adjoining tissues such as bone or subcutaneous tissues, or arise from previous septicemia, the term “secondary pyomyositis” is more appropriate to define disease pathogenesis.2
Staphylococcal pyomyositis is clinically divided into three consecutive stages: the invasive stage, with local symptoms and low-grade fever, the suppurative stage, with abscess formation, and the late stage, with dissemination of the infection, if the abscess remains untreated in the previous stages.2,3 Bacteriemia, sepsis, acute renal failure, ARDS, and metastatic abscesses are some of the complications that have been described.2,15 Septic pulmonary emboli are usually associated with right-sided endocarditis or deep vein thrombosis/thrombophlebitis. However, even in the absence of profound intravascular sources, septic pulmonary emboli may represent metastatic abscesses to the lungs arising from primary deep tissue infections such as osteomyelitis, septic arthritis, and rarely pyomyositis.15
A key feature of staphylococcal skin and soft tissue infections is recurrence, which is estimated to occur in approximately 30% of all cases.16 These cases include both relapses, which refer to incompletely treated primary episodes that result from the emergence of the original microorganism, or re-infections, which describe infections with a new microorganism.17 Traditionally, a threshold of 6-month time interval has been used to distinguish clinically relapse from re-infection, although only molecular fingerprinting of the isolated bacteria can provide definite discrimination.17
In addition to the well-substantiated staphylococcal recurrence, there are several reports of invasive staphylococcal infections in the literature – primarily osteomyelitis – where staphylococcus remained surprisingly latent for a very long period of time – over a decade or decades.8–14 These observations were reported arbitrarily as staphylococcal reactivations, and not as simple relapsing, persistent or recurrent infections. In the case of osteomyelitis, it had been taught that “osteomyelitis which had its onset in the pre-penicillin era could never be considered cured”.9
At that time, authors were not able to provide solid evidence or explain the exact pathophysiological mechanisms of this staphylococcal latency. Later it was shown that S. aureus has the ability to transform into an atypical intracellular pathogen. Similarly to the bacteria embedded in biofilms, when internalized, staphylococcus can change its characteristics, as to remain metabolically inactive or to decrease its susceptibility to certain antibiotics. In the experimental study by Krut et al.,18 it was shown that only rifampicin was able to eliminate completely intracellular S. aureus from non-phagocytic cells, while linezolid, clindamycin, and azithromycin induced a state of intracellular persistence, and vancomycin failed to prevent host cells from dying. Furthermore, in order to explain staphylococcal dormancy, research has been focused on the role of the Accessory Gene Regulator (AGR) system. In particular, activation of the AGR system is responsible for the synthesis of virulence factors, such as exoproteins/cytotoxins, leading to the subsequent development of the abscess lesion and bacterial survival. Investigating the AGR activation kinetics, Wright et al. reported an “eclipse phase” which probably represents a metabolic shut-down of virulent strains although their intracellular survival remained intact.19 This hypothesis may in part explain how Staphylococcus can remain latent intracellularly for long periods of time, but it does not elucidate what happens during reactivation. In this setting, none of the authors reporting cases of late reactivations was able to provide any possible explanation or suggest precipitating factors, as all reactivations occurred unexpectedly in previously healthy, immunecompetent patients without evidence of recent trauma or skin contamination.
Certainly, one could argue that the above mentioned cases represent re-infections and not reactivations. In fact, Uçkay et al.20 reported three cases of recurrent osteomyelitis caused by different bacterial strains or other bacteria, suggesting that formerly infected and altered bone surface might present a region of diminished resistance for a new infection. On the other hand, in another case of recurrent osteomyelitis after 75 years8 an old sinus tract during surgery was found, which had not been drained in the first place. Cultures from the bone and tract grew only S. aureus, which was sensitive, as expected, to all antibiotics. Investigators proceeded to sequence typing, which placed the isolated strain among the ST30 S. aureus clone, believed to have been spread throughout the world during the 1950s and 1960s, and, therefore, provided evidence of staphylococcal reactivated osteomyelitis.
In our case, this identification could not have been performed. As no medical records were available, we only had a history of a staphylococcal osteomyelitis when the patient was six years old for which she was operated on. Administration of antibiotics in the mid-1950s in Greece is also questionable. Reactivation of staphylococcal infection was postulated because of the following:
- (i)
The patient was previously healthy and immunecompetent, and no predisposing factors were identified.
- (ii)
Cultures from nostrils and skin folds turned out negative for S. aureus, rejecting the hypothesis of previous colonization.
- (iii)
The patient presented with a two-week history of non-specific symptoms – time consistent with early stages of primary pyomyositis – until she developed the septic complications of the late stage.
- (iv)
Recurrence of staphylococcal infection began in the same anatomical region (left ilium) and possibly expanded into the neighboring muscle groups as abscesses of the left ileopsoas and gluteal muscles.
- (v)
Septic pulmonary emboli arising from primary pyomyositis were consistent with the absence of both right-sided endocarditis and thrombosis of the lower extremities.
- (vi)
Pus and blood cultures identified a methicillin-sensitive staphylococcal strain, although incidence rates of MRSA in Greece are amongst the highest in Europe, estimated to be over 40%.
The main counter argument to our primary hypothesis that this case represents a reactivated staphylococcal soft tissue infection is that there was no evidence of osteomyelitis in the present MRI. As in the report of Stevens et al.,10 recurrence of hip osteomyelitis with secondary pyomyositis of the adjoining muscles would be a probable case scenario that could explain staphylococcal reactivation. Instead, our findings suggest that either MRI was unable to show an underlying osteomyelitis or that Staphylococcus remained quiescent from previous seeding into micro-abscesses of the surrounding muscles. If this is the case, that makes our report the first case of reactivated staphylococcal pyomyositis.
ConclusionStaphylococcal pyomyositis is a dangerous infection with high mortality that is increasingly being recognized even in temperate climates. Diagnosis should be immediate and management should be aggressive, in order to prevent sepsis and spread of metastatic abscesses. If complete eradication fails, recurrence manifesting either as relapse or even as reactivation is to be anticipated. Finally, future studies should focus on the pathophysiology of staphylococcal latency and the precipitating factors that may lead to its reactivation.
Conflicts of interestThe authors declare no conflicts of interest.