This study aimed to evaluate the prevalence of sexually transmitted infections (STIs) and associated risk factors in HIV-infected pregnant women followed for prenatal care in Salvador, Bahia. This was a cross-sectional study of 63 women seeking prenatal care at a reference center. Participants were interviewed regarding socio-epidemiological and clinical history, and were tested for HBsAg, anti-HCV, anti HTLV I/II, VDRL, Chlamydia trachomatis, Neisseria gonorrhoeae, Mycoplasma hominis, Ureaplasma urealyticum, CD4 count, and HIV plasma viral load. The main outcome variable was the presence of any STI. The mean age of patients was 28.2 years (16-40 years). 23 (36.5%) were diagnosed with at least one STI. The frequency of diagnoses was: HBV, 3.2%; HCV, 8.1%; HTLV I/II, 3.4%; syphilis, 9.5%; Chlamydia trachomatis, 11.1%; HPV, 15.0%; Mycoplasma hominis, 2.1%, and Ureaplasma urealyticum, 2.1%. No case of Neisseria gonorrhoeae was identified. No association was found between socio-epidemiological variables and the presence of an STI. CD4 T lymphocyte < 500 cells/μL (p=0.047) and plasma viral load >1,000 copies (p = 0.027) were associated with the presence of STI. STIs are frequent in pregnant women infected with HIV, and all HIV-infected pregnant women should be screened to decrease transmission of these pathogens and to protect their own health.
Sexually transmitted infections (STIs) have been shown to increase the risk of acquiring and transmitting human immunodeficiency virus (HIV) infection.1 In women this association has been principally observed in the presence of genital ulcer disease and cervicitis, which disrupt the epithelial barrier, cause local inflammation, and increase genital tract viral load.1,2
Pregnant women are at increased risk of STIs due to physiological changes that accompany pregnancy, such as congestion of the cervix, edema of the vaginal mucosa, and alterations in the vaginal flora.3 Additionally, pregnant women may be less likely to have partners that use condoms, and may have fewer options to leave unsafe relationships.4 STI in pregnancy is associated with increased neonatal morbidity and mortality and can cause premature labor, low birth weight, stillbirth, premature rupture of membranes, and severe congenital infections.5 In pregnant women infected with HIV, STI co-infection can increase the risk of vertical transmission of HIV and other pathogens.6 This risk is particularly pronounced if the HIV infection is acquired during pregnancy.7
Some STIs are curable, and treatment can be administered during pregnancy.8 Viral infections, such as hepatitis B and C, human papillomavirus (HPV) and HIV itself, although not curable, can be treated, and there are measures to control viremia that can be implemented during pregnancy. Early diagnosis of these infections can make a difference in the evolution of pregnancy and prognosis.5
In Brazil, several studies have estimated the prevalence of STIs in low- or medium-risk pregnant women, but there are few data on the prevalence of STIs among women already infected with HIV.9 The prevalence of STIs and associated risk factors were evaluated in pregnant HIV-infected women at a public-sector specialty clinic in Salvador, Bahia, Brazil.
MethodsThis was a cross-sectional study at the Centro Estadual Especializado em Diagnóstico, Assistência e Pesquisa (CEDAP) in Salvador, capital of the state of Bahia, conducted from October 1, 2010 to September 30, 2011. CEDAP is the main reference center for high-risk prenatal care of HIV-infected pregnant women in Salvador. Approximately 85 pregnant women attend the CEDAP annually.
The Research Ethics Committee of the Bahia State Health Secretary (Comitê de Ética em Pesquisa da Secretaria de Saúde do Estado da Bahia (CEP/SESAB) reviewed and approved the study (protocol number 25/2010).
HIV-infected pregnant women attending CEDAP during the study period who agreed to participate were included. Patients who had used antibiotics in the prior 30 days, who presented with obstetrical complications at the first visit, such as threatened abortion, premature labor or premature rupture of the membranes, and those who had only one consultation were excluded from the study.
Data were collected at the first prenatal consultation in a private interview room. The participants of the study signed an informed consent. Questionnaires included socio-demographic, economic and clinical data, which included gestational age; age at first pregnancy; age at first intercourse; date of HIV diagnosis; alcohol, tobacco or drug use; number of sexual partners and prior history of STI. Patients were evaluated according to Ministry of Health's guidelines;10 during the physical examination, cervicovaginal smears were collected for STI diagnosis and cervical cytology. During the same visit, blood was drawn for serological tests, CD4 cell count, and plasma viral load determination. All patients were treated syndromically for STIs during the first prenatal visit, and were subsequently treated based on laboratory diagnoses.
Patients’ serum was tested using commercially available kits for hepatitis B surface antigen (HBsAg) (ETI-MAK-4, Diasorin - São Paulo, Brazil), hepatitis C antibody (HCV) (Murex, Generlabs Diagnostic - São Paulo, Brazil), and antibodies to human T-lymphotropic virus types I and II (HTLV I/II) (Symbiosis, ALKA Tecnologia - São Paulo, Brazil). VDRL (Wama - São Carlos, Brazil) was used to screen for syphilis and titered results were provided. CD4 cell levels were determined using flow cytometry (Facscalibur, Becton Dickinson - California, USA); plasma viral load was determined using VERSANT RNA 3.0 (Siemens Health Care Diagnostics - Munich, Germany). Endocervical specimens were cultured on Thayer-Martin medium for Neisseria gonorrhoeae, and on A7B and U10 culture media for Mycoplasma hominis and Ureaplasma urealyticum. Endocervical specimens were also tested for Chlamydia trachomatis using hybrid capture techniques (QIAGEN – São Paulo, Brazil). Cytological alterations corresponding to viral infection by HPV were identified by Papanicolaou smear.
The primary outcome variables were presence of a positive anti-HTLV I/II, AgHbs, anti-HCV, VDRL; positive capture hybrid for Chlamydia trachomatis; positive culture for Neisseria gonorrheae, Mycoplasma hominis or Ureaplasma urealyticum or abnormal Pap smear. An outcome variable was constructed for any positive STI test. Associations between predictor variables and both the composite outcome variables were evaluated using the chi-squared test, Fisher's exact test, and, for continuous variables, Student's t-test. Continuous variables were characterized by means and standard deviations for normally distributed variables, and by medians and interquartile ranges for variables that were not normally distributed. The Statistical Package for Social Sciences (SPSS) version 17.0 (SPSS Inc. – Chicago, USA) was used for all statistical testing.
ResultsThis study evaluated 63 pregnant women infected with HIV. The mean age of participants was 28.2 years (range 16-40 years); five (8.1%) were 18 years of age or less. 51 (82.3%) participants reported that they were married or in a stable relationship. The large majority (95.2%) of patients self-identified as being either of African or mixed ancestry. The mean gestational age at first consultation was 19 weeks. Overall, the mean CD4 count was 563 cells/μL, and the median plasma viral load was 2,651 copies/mL. 19 (30.2%) participants had fewer than 350 cells/μL. 25 (39.7%) had plasma viral loads fewer than 1,000 copies/mL, and 24 (38.1%) were on antiretroviral therapy before pregnancy.
Patients reported a variety of risk factors. 24 (38.1%) reported alcohol use, of whom 20 (31.7%) reported drinking alcohol only on weekends, three (4.8%) during the week, and one (1.6%) daily. The large majority of participants (55, 87.3%) reported not smoking, and ten (15.9%) reported using non-parenteral illicit drugs (marijuana, crack, or cocaine). No patient reported injectable drug use. The mean age at first intercourse was 15.2 years, and almost one-third of participants began having sexual intercourse at 14 years of age or less. 23 (37.1%) had had their first pregnancy at 18 years of age or younger. The lifetime reported number of sexual partners ranged from one to 500; 34.5% reported three or fewer lifetime partners, and 34.5% reported four to nine. 28 (44.4%) participants also reported a prior history of STI.
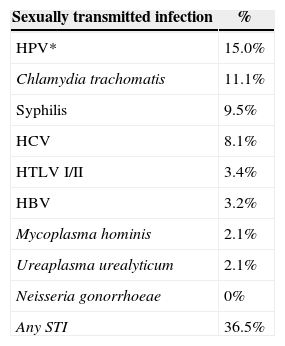
Evidence of STI was found in 23 (36.5%) patients. Regarding STI from serological results, prevalence of 3.2% (2/63) of HBsAg positive, 8.1% (5/62) anti-HCV, 9.5% (6/63) of VDRL reagent, and 3.4% (2/59) anti-HTLV reagent were found. For cervicitis due to Chlamydia trachomatis diagnosed through hybrid capture, 11.1% (7/63) were positive, 2.1% (1/48) of cultures were positive for Mycoplasma hominis, 2.1% (1/48) were positive for Ureaplasma urealyticum, and there were no positive results for Neisseria gonorrhoeae. The presence of cytological alterations, which were described as low-grade squamous intraepithelial lesion, was observed in 15.0% (9/60) patients (Table 1). Six (9.5%) patients had more than one STI.
Prevalence of STI in HIV-infected pregnant women, CEDAP, Salvador, BA, Brazil, 2010-2011.
| Sexually transmitted infection | % |
|---|---|
| HPV* | 15.0% |
| Chlamydia trachomatis | 11.1% |
| Syphilis | 9.5% |
| HCV | 8.1% |
| HTLV I/II | 3.4% |
| HBV | 3.2% |
| Mycoplasma hominis | 2.1% |
| Ureaplasma urealyticum | 2.1% |
| Neisseria gonorrhoeae | 0% |
| Any STI | 36.5% |
CEDAP, Centro Estadual Especializada em Diagnóstico, Assistência e Pesquisa; *Cytological alterations – low-grade squamous intraepithelial lesion.
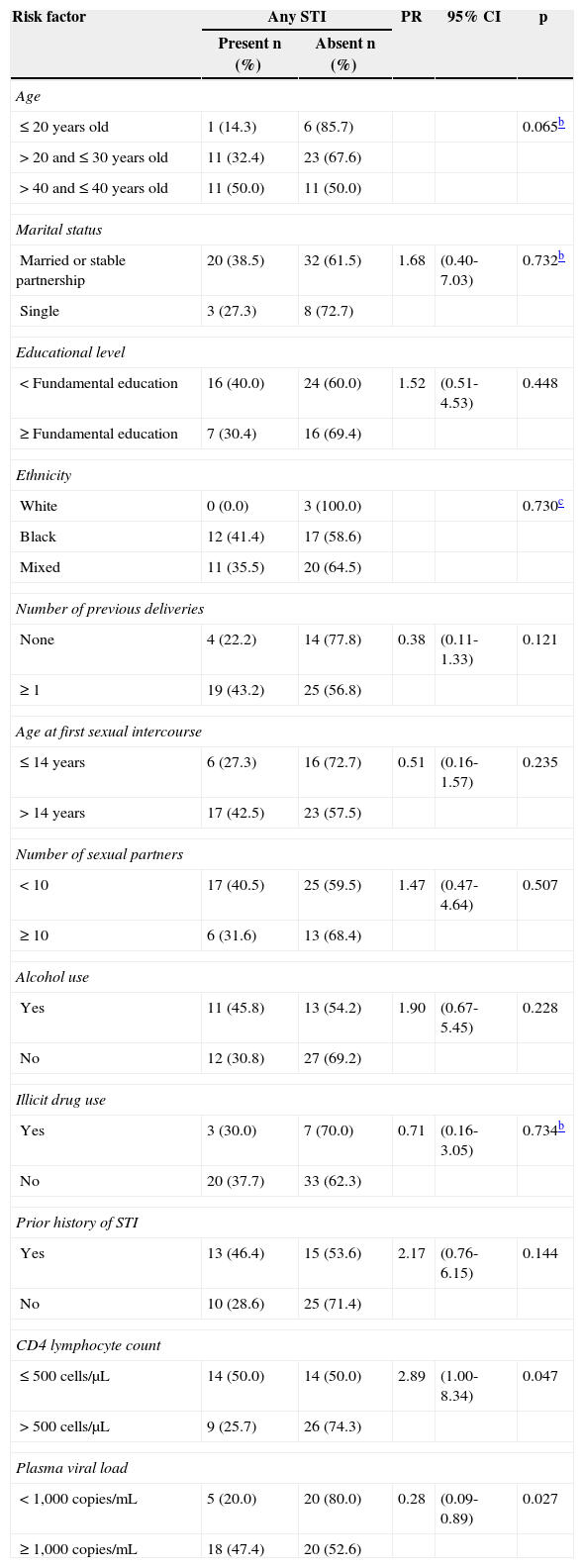
No association was found between socio-epidemiological variables and presence of STI. CD4 T lymphocyte count < 500 cells/μL (p=0.047) and plasma viral load > 1000 copies/mL (p=0.027) were associated with the presence of an STI (Table 2).
Risk factors for acute and chronic STIa by demographic and clinical factors, HIV-infected pregnant women, CEDAP, Salvador, BA, Brazil, 2010–2011.
| Risk factor | Any STI | PR | 95% CI | p | |
|---|---|---|---|---|---|
| Present n (%) | Absent n (%) | ||||
| Age | |||||
| ≤ 20 years old | 1 (14.3) | 6 (85.7) | 0.065b | ||
| > 20 and ≤ 30 years old | 11 (32.4) | 23 (67.6) | |||
| > 40 and ≤ 40 years old | 11 (50.0) | 11 (50.0) | |||
| Marital status | |||||
| Married or stable partnership | 20 (38.5) | 32 (61.5) | 1.68 | (0.40-7.03) | 0.732b |
| Single | 3 (27.3) | 8 (72.7) | |||
| Educational level | |||||
| < Fundamental education | 16 (40.0) | 24 (60.0) | 1.52 | (0.51-4.53) | 0.448 |
| ≥ Fundamental education | 7 (30.4) | 16 (69.4) | |||
| Ethnicity | |||||
| White | 0 (0.0) | 3 (100.0) | 0.730c | ||
| Black | 12 (41.4) | 17 (58.6) | |||
| Mixed | 11 (35.5) | 20 (64.5) | |||
| Number of previous deliveries | |||||
| None | 4 (22.2) | 14 (77.8) | 0.38 | (0.11-1.33) | 0.121 |
| ≥ 1 | 19 (43.2) | 25 (56.8) | |||
| Age at first sexual intercourse | |||||
| ≤ 14 years | 6 (27.3) | 16 (72.7) | 0.51 | (0.16-1.57) | 0.235 |
| > 14 years | 17 (42.5) | 23 (57.5) | |||
| Number of sexual partners | |||||
| < 10 | 17 (40.5) | 25 (59.5) | 1.47 | (0.47-4.64) | 0.507 |
| ≥ 10 | 6 (31.6) | 13 (68.4) | |||
| Alcohol use | |||||
| Yes | 11 (45.8) | 13 (54.2) | 1.90 | (0.67-5.45) | 0.228 |
| No | 12 (30.8) | 27 (69.2) | |||
| Illicit drug use | |||||
| Yes | 3 (30.0) | 7 (70.0) | 0.71 | (0.16-3.05) | 0.734b |
| No | 20 (37.7) | 33 (62.3) | |||
| Prior history of STI | |||||
| Yes | 13 (46.4) | 15 (53.6) | 2.17 | (0.76-6.15) | 0.144 |
| No | 10 (28.6) | 25 (71.4) | |||
| CD4 lymphocyte count | |||||
| ≤ 500 cells/μL | 14 (50.0) | 14 (50.0) | 2.89 | (1.00-8.34) | 0.047 |
| > 500 cells/μL | 9 (25.7) | 26 (74.3) | |||
| Plasma viral load | |||||
| < 1,000 copies/mL | 5 (20.0) | 20 (80.0) | 0.28 | (0.09-0.89) | 0.027 |
| ≥ 1,000 copies/mL | 18 (47.4) | 20 (52.6) | |||
CEDAP, CEDAP, Centro Estadual Especializada em Diagnóstico, Assistência e Pesquisa; PR, prevalence ratio; 95% CI, 95% confidence interval.
36.5% of HIV-infected pregnant women in our sample were found to have another STI. This is an enormous STI burden in a highly vulnerable population. The prevalence of infection with individual agents found in this study was in general substantially higher than that reported in the general population of pregnant women in Brazil, as 0.88% for HTLV I/II,11 1.8% for hepatitis B,12 1.4% for HCV,13 3.9% for syphilis,11 9.8% for C. trachomatis14 and 1.5% for N. gonorrhoeae.15 However, a recent study on a population of pregnant women with HIV infection in Southern Brazil found that 50% of women were infected with HBV, HCV, syphilis, or HPV.16 Rates of bacterial STI similar to those found in the present study were reported in a study conducted in Kenya, but in that study the investigators also reported very high prevalence of bacterial vaginosis and trichomoniasis (37% and 16%, respectively), which were not assessed in the present study.17 In Europe, 24.5% of pregnant HIV-infected women had one or more STI; syphilis was the most common bacterial STI (2.0%), and HPV-related warts were found in 8.6% in that study.18 These findings alone reinforce the need for early diagnosis of STI in this population to avoid additional obstetrical and neonatal complications.
In this population, no association of STI with the risk factors considered associated with the presence of STI was found, such as sexual activity at ≤ 14 years (p = 0.235), more than ten sexual partners (p = 0.507), alcohol use (p= 0.228), illicit drug use (p = 0.734), and previous history of STI (p = 0.144), which have been previously found in HIV-uninfected women.19 The lack of association with socio-demographic and economic factors suggest that there is not a specific subgroup that should be evaluated; all HIV-infected pregnant women are at increased risk for STI.
In contrast to other studies, the principal risk factor for STI found in this study was having a CD4 lymphocytes count < 500 cells/μL (p=0.047) and an elevated HIV plasma viral load (p = 0.027).17 It is possible that this finding of high STI prevalence may represent failure of a deteriorating immune system to clear some of these infections. This association was found by other authors,18 suggesting that compromised immunity associated with the presence of STI could also be explained by the difficulty of obtaining early diagnosis and appropriate treatment. 17.5% of pregnant women in this study had CD4 counts <200 cells/μL, which is the definition of acquired immunodeficiency syndrome (AIDS) according to the Centers for Disease Control and Prevention (CDC, USA) criteria;20 this is a strong indication of poor access and inadequate monitoring.
The present study has some limitations. First, it was difficult to enroll women in the present study; many found a speculum examination during pregnancy to be culturally unacceptable. Some women did not return to continue prenatal care. This potentially created non-participation and selection bias, and also may have led to a loss of statistical power. Moreover, while hepatitis B, hepatitis C, and HTLV I/II were classified as STIs, they are also transmitted parenterally and during labor and delivery; however, these routes of transmission were evaluated. Finally, this study was conducted in a public clinic in a city in Northeastern Brazil, with a small number of pregnant women; how these findings will generalize to the rest of the country is not clear.
ConclusionA high STI burden was found in pregnant HIV-infected women. There is a clear need for early diagnosis of these infections in pregnancy, in order to prevent complications and vertical transmission. It is suggested that additional large studies be conducted in other regions of Brazil in order to understand the epidemiology of STIs in this specific population, and to implement the most effective prevention policies based on these findings.
Conflict of interestAll authors declare to have no conflict of interest.
The authors thank the University of California, San Francisco, Center for AIDS Prevention Studies, US National Institute of Mental Health (NIMH), P30 MH062246, the International Traineeships in AIDS Prevention Studies, US NIMH, R25MH064712; and the Fogarty International Center's International, Operational and Health Services Research Training Award, Brazilian Scientists Program (D43 TW005799).