A cross-sectional study on prevalence of HBV and HDV infection, risk factors and genotype distribution of HBV infection was conducted among 848 HIV-infected patients in Mato Grosso do Sul, Central Brazil.
MethodsSerum samples of 848 participants were tested for hepatitis B surface antigen (HBsAg), hepatitis B core antibody (anti-HBc) and hepatitis surface antibody (anti-HBs). HBsAg positive samples were tested for anti-HBc IgM, HBeAg, anti-HBe, anti-HCV, and total anti-HDV. HBsAg and anti-HBc positive were subjected to DNA extraction. Viral DNA was amplified by semi-nested PCR for the regions pre-S/S and then purified and genotyped/subgenotyped by direct sequencing. Student's t-test, chi-square test and Fisher's exact test were used to compare variables and to evaluate association between HBV positivity (defined as anti-HBc and/or HBsAg positivity) and risk factors.
ResultsAmong the 848 HIV infected patients investigated 222 had serological markers of HBV infection. The prevalence rate of HIV-HBV coinfection was 2.5% (21/848; 95% CI: 1.4–3.5%); 484 (57.1%) patients were susceptible for HBV infection. There were no cases of anti-HDV positive and only one (0.1%) anti-HCV-positive case among the HIV-HBV coinfected patients. Male gender, increasing age, family history of hepatitis, use of illicit drug, and homosexual activity were independent factors associated with HBV exposure. The phylogenetic analysis based on the S gene region revealed the presence of genotypes D (76.9%), F (15.4%) and A (7.7%) in the study sample.
ConclusionThis study demonstrates the low prevalence of HIV-HBV infection and also highlights the need for early vaccination against HBV as well as testing for HBV, HCV and HDV in all HIV-infected individuals.
An estimated 34 million people are currently infected with HIV worldwide, and of these, three million are chronically infected with HBV and seven million are coinfected with HCV due to the similarity in the transmission routes.1
However, many factors can influence and modify these estimates, such as geographical region, risk groups, age of infection, modes of transmission efficacy or efficiency of exposure. Global prevalence of HIV-HBV coinfection is heterogeneous, ranging from 6% to 26%.1,2 In the Central-West Region of Brazil, scarce information is available on the prevalence of HIV-HBV coinfection and HDV infection. Most of the studies, conducted in South and Southeast regions of Brazil, have reported HIV-HBV coinfection prevalence, ranging from 2.3% to 27.3%.3–7 Furthermore, this is the first study reporting the HBV genotypes in HIV infected individuals in Central-West Region of Brazil.
The survival of HIV infected patients has markedly improved since the introduction of highly active antiretroviral therapy (HAART) and deaths from AIDS-related causes have declined. However, multiple studies have shown that liver disease, caused by hepatitis B virus (HBV) and hepatitis C virus (HCV) coinfections, chronic alcoholism, hepatic tuberculosis or hepatotoxicity associated with antiretroviral therapy, has emerged as the leading cause of mortality.8 Evidence has demonstrated that coinfection with HIV significantly alters the natural history of HBV infection, influencing disease progression.9,10 HBV coinfection negatively influences the course of HIV infection but this hypothesis remains quite controversial. The mechanisms by which HBV may influence HIV infection are poorly understood.9,11
Several viral factors, including HBV genotype and specific viral mutations, have been documented to influence the clinical outcome of HBV infection.12 While HBV genotype A is the most common in co-infected individuals, the non-A genotypes are associated with more advanced fibrosis. Furthermore, genotypes B and C were associated with higher viral loads in comparison to genotypes A and D.10 Conditions of selection pressures including vaccination, antiviral therapy and host immune response may result in the emergence of viral variants which may be associated with progression to more severe liver disease.12
Hepatitis Delta virus (HDV) infection has a worldwide distribution and infects human already infected by Hepatitis B virus (HBV). Approximately, 15 million people worldwide are infected with HDV and the prevalence of HDV infection varies greatly throughout different geographic regions. In Brazil, HDV is highly endemic in the Amazonian region, but data in other regions from Brazil are scarce.13,14
This study aimed to determine the prevalence of HBV and HDV infection, HIV-HBV coinfection and HBV genotype distribution in HIV-infected patients at a referral center for HIV/AIDS, in Campo Grande, Mato Grosso do Sul, Central-West Region of Brazil. Additionally, epidemiological data regarding risk factors and vaccinal status was assessed.
Materials and methodsStudy populationFor this cross-sectional study, 848 HIV infected patients were recruited from the Esterina Corsini University Hospital of the Federal University of Mato Grosso do Sul (HU/UFMS) and from the Reference Center of Infectious and Parasitic Diseases (CEDIP) in the capital city of Campo Grande, Mato Grosso do Sul, Central-West Region of Brazil, between November 2009 and July 2011. The age of patients ranged from 14 to 87 years with a mean of 41.6 years; 483 (57%) were men and 365 (43%) were women. Participants were classified as HIV-HBV coinfected if they presented hepatitis B surface antigen (HBsAg) positive. Serological evidence of exposure to HBV was defined as anti-HBc positivity. Individuals with previous serological evidence of HBV vaccination (anti-HBs alone) were excluded from the HBV risk factors analysis.
HIV-positive patients, who consented to participate in the investigation by signing the Instrument of Consent, were interviewed using a standard form, which collected information regarding their sociodemographic data and risk characteristics associated with HIV-HBV coinfection. The interviews were conducted individually to guarantee full privacy of the research participants. Blood samples were collected from all individuals and sera were stored at −20°C. This study was approved by the Ethics Committee (CEP) of the Federal University of Mato Grosso do Sul.
Serologic testsSerum samples of 848 participants were tested for hepatitis B surface antigen (HBsAg), hepatitis B core antibody (anti-HBc) and hepatitis surface antibody (anti-HBs) using enzyme-linked immunosorbent assay (ELISA) (bioMérieux BV, Boxtel, The Netherlands). Patients with results initially reactive to anti-HBc or HBsAg were subjected to new blood collection and sera were tested in duplicate. HBsAg positive samples were tested for anti-HBc IgM, HBeAg, anti-HBe, anti-HCV and total anti-HDV by electrochemiluminescence immunoassay (ECLIA), using the Cobas® e601 analyzer (Roche Diagnostics, Mannheim, Germany), according to manufacturer's instructions.
DNA extraction and amplificationHBsAg positive samples were submitted to DNA extraction using the High Pure Viral Nucleic Acid Kit (Roche, Mannheim, Germany). After precipitation, the pellet was dried and resuspended in 50μL of elution buffer. The pre-S/S genome region was partially amplified by semi-nested PCR. The primers used in the first round were PS1 (5′-CCATATTCTTGGGAACAAGA-3′, nucleotide position 2826-2845) and P3 (5′-AAAGCCCAAAAGACCCACAA-3′, nucleotide position 1019-1000). The first round of amplification was performed with 1μL of DNA and one unit of Taq DNA polymerase (Invitrogen, San Diego, CA, USA) in a final volume of 25μL, under the following conditions: after an initial denaturation step of 2min at 94°C, DNA was amplified using 30 cycles of 94°C for 30s, 55°C for 30s, and 72°C for 1min and 30s, followed by a final elongation step for 7min at 72°C. The second round was conducted by using one sense primer (PS1) and two antisense primers S2 (5′-GGGTTTAAATGTATACCCAAAA-3′, nucleotide position 819-841), and S22 (5′-GTATTTAAATGGATACCCACAGA-3′, 819-841) located at the same position on the genome to facilitate the amplification of all HBV genotypes. The second round of amplification was performed in a final volume of 50μL, using 1μL of the first round PCR product, under the following conditions: an initial denaturation step of 3min at 94°C, followed by 30 cycles of 95°C for 30s, 52°C for 40s, and 72°C for 2min, followed by a final elongation step for 7min at 72°C. Ten microliters of amplification product (∼1200bp in length) was loaded on 2% agarose gels, electrophoresed, stained with ethidium bromide and visualized under UV light.
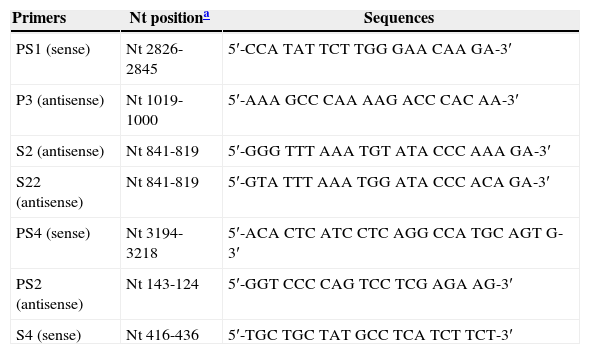
Nucleotide sequencing and phylogenetic analysisNucleotide sequences of the pre S/S region were determined by direct sequencing with BigDye Terminator kit (Applied Biosystems, CA, USA) using specific HBV primers, PS1, PS4, S4, S2 and PS2 (Table 1). Sequencing reactions were analyzed on an ABI3730 automated sequencer (Applied Biosystems). HBV sequences determined in this study were deposited in GenBank database under accession numbers KF 111239 to KF 111245. Multiple sequence alignment was performed with CLUSTAL X program (version 1.63b, December 1997; http://www-igbmc.u-strasbg.fr/BioInfo/ClustalX/) after addition of reference sequences representing all known HBV subgenotypes available at the GenBank. Phylogenetic analysis was performed by Bayesian Inference using Monte Carlo Markov Chain (MCMC) in MrBayes version 3.1.2 algorithm (http://mrbayes.csit.fsu.edu) under the model of nucleotide substitution GTR+Γ+I, selected as the best-fit model by jModeltest program (http://darwin.uvigo.es/software/jmodeltest.html). MCMC algorithm was implemented in two independent runs for 1×107 generations with a sample frequency of 100 and a burn-in of 25% of the initial trees. Chains convergence was confirmed by calculation of Effective Sample Size (ESS) values >200 using TRACER v1.5 (http://tree.bio.ed.ac.uk/software/tracer/). The phylogenetic tree was visualized using FigTree v1.3.1 (http://tree.bio.ed.ac.uk/software/figtree/).
Primers used for PCR amplification and sequencing.
| Primers | Nt positiona | Sequences |
|---|---|---|
| PS1 (sense) | Nt 2826-2845 | 5′-CCA TAT TCT TGG GAA CAA GA-3′ |
| P3 (antisense) | Nt 1019-1000 | 5′-AAA GCC CAA AAG ACC CAC AA-3′ |
| S2 (antisense) | Nt 841-819 | 5′-GGG TTT AAA TGT ATA CCC AAA GA-3′ |
| S22 (antisense) | Nt 841-819 | 5′-GTA TTT AAA TGG ATA CCC ACA GA-3′ |
| PS4 (sense) | Nt 3194-3218 | 5′-ACA CTC ATC CTC AGG CCA TGC AGT G-3′ |
| PS2 (antisense) | Nt 143-124 | 5′-GGT CCC CAG TCC TCG AGA AG-3′ |
| S4 (sense) | Nt 416-436 | 5′-TGC TGC TAT GCC TCA TCT TCT-3′ |
Prevalence data and 95% confidence intervals were calculated. Student's t-test (continuous variable), chi-square test and Fisher's exact test (categorical variables) were used to compare variables and to evaluate association between HBV infection. For the purposes of analysis, a positive identification of HBsAg and/or anti-HBc markers was considered an indication of current or previous HBV infection. Vaccinated individuals (only anti-HBs reagent; n=142) were not included in the HBV risk factors analysis. These risk factors estimated by odds ratio in univariate analysis were further analyzed in a stepwise logistic regression model to identify possible confounders. Differences were considered statistically significant when p-values<0.05. Statistical evaluations were performed using EpiInfo (version 3.5.3; http://wwwn.cdc.gov/epiinfo/) and SPSS (version 11.0; SPSS Inc., Chicago, USA, 1999).
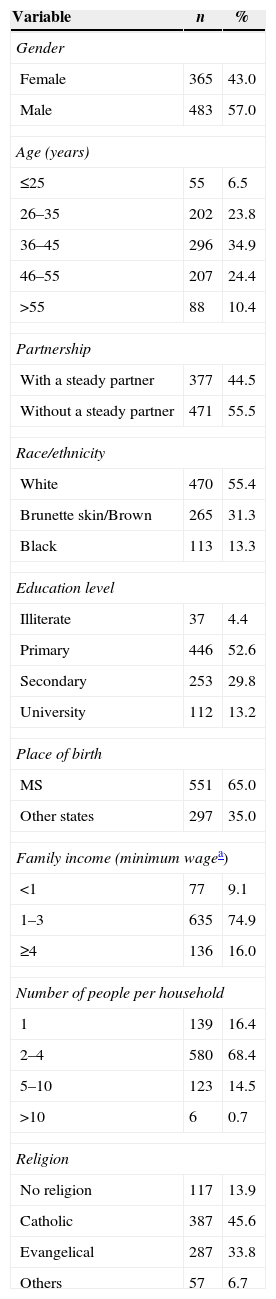
ResultsA total of 848 patients HIV-infected were included in the study. The sociodemographic characteristics of the all patients are summarized in Table 2.
Sociodemographic characteristics among HIV-infected patients in Campo Grande, Brazil, 2013 (n=848).
| Variable | n | % |
|---|---|---|
| Gender | ||
| Female | 365 | 43.0 |
| Male | 483 | 57.0 |
| Age (years) | ||
| ≤25 | 55 | 6.5 |
| 26–35 | 202 | 23.8 |
| 36–45 | 296 | 34.9 |
| 46–55 | 207 | 24.4 |
| >55 | 88 | 10.4 |
| Partnership | ||
| With a steady partner | 377 | 44.5 |
| Without a steady partner | 471 | 55.5 |
| Race/ethnicity | ||
| White | 470 | 55.4 |
| Brunette skin/Brown | 265 | 31.3 |
| Black | 113 | 13.3 |
| Education level | ||
| Illiterate | 37 | 4.4 |
| Primary | 446 | 52.6 |
| Secondary | 253 | 29.8 |
| University | 112 | 13.2 |
| Place of birth | ||
| MS | 551 | 65.0 |
| Other states | 297 | 35.0 |
| Family income (minimum wagea) | ||
| <1 | 77 | 9.1 |
| 1–3 | 635 | 74.9 |
| ≥4 | 136 | 16.0 |
| Number of people per household | ||
| 1 | 139 | 16.4 |
| 2–4 | 580 | 68.4 |
| 5–10 | 123 | 14.5 |
| >10 | 6 | 0.7 |
| Religion | ||
| No religion | 117 | 13.9 |
| Catholic | 387 | 45.6 |
| Evangelical | 287 | 33.8 |
| Others | 57 | 6.7 |
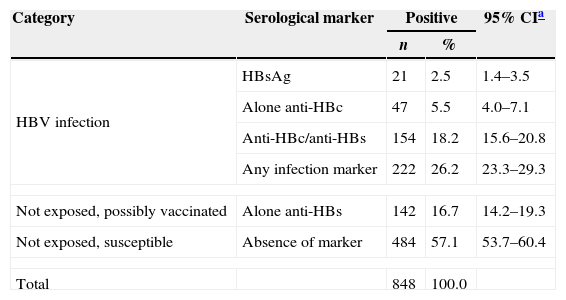
Among the 848 HIV infected patients investigated, 222 (26.2%; 95% CI: 23.3–29.3%) presented serological markers of HBV infection. Among them, 21 (2.5%) were HBsAg positive, 154 (18.1%) had anti-HBc and anti-HBs markers and 47 (5.5%) were anti-HBc alone.
The prevalence rate of HBV-HIV coinfection was 2.5% (21/848; 95% CI: 1.4–3.5%) and among these 21 were HBsAg positive samples, 3 (14.3%) were HBsAg isolated, HBeAg was detected in 7 (33.3%), anti-HBe in 9 (42.9%), while 5 (23.8%) were negative for both HBeAg and anti-HBe. Only 1 sample was HBsAg plus anti-HBc IgM.
The immune response to HBV vaccine (anti-HBs alone) among HIV-1 infected individuals was present in 16.7% of the patients (142/848) (Table 3). Data on vaccination history was available for 385 HIV-infected participants. Only 169 of them had completed the HBV vaccination schedule (3 doses). Among them, 141 had no infection marker (anti-HBc or HBsAg). Immune response to HBV vaccine (anti-HBs alone) was found in 43.2% (61/141) of these individuals.
Prevalence of serological markers of HBV infection in HIV-infected patients in Campo Grande, Mato Grosso do Sul, Central-West Brazil.
| Category | Serological marker | Positive | 95% CIa | |
|---|---|---|---|---|
| n | % | |||
| HBV infection | HBsAg | 21 | 2.5 | 1.4–3.5 |
| Alone anti-HBc | 47 | 5.5 | 4.0–7.1 | |
| Anti-HBc/anti-HBs | 154 | 18.2 | 15.6–20.8 | |
| Any infection marker | 222 | 26.2 | 23.3–29.3 | |
| Not exposed, possibly vaccinated | Alone anti-HBs | 142 | 16.7 | 14.2–19.3 |
| Not exposed, susceptible | Absence of marker | 484 | 57.1 | 53.7–60.4 |
| Total | 848 | 100.0 | ||
Among 848 patients, 484 (57.1%) were susceptible for HBV infection. Of them, 77 patients (15.9%) received the complete vaccination schedule against hepatitis B.
Only one HIV infected patient (0.1%) had both HBV (HBsAg plus total anti-HBc positive) and HCV (anti-HCV) coinfection. Among 21 HBsAg positive patients, none presented anti-HDV positivity.
The prevalence of HBV serological markers was higher in men (39.1%) than in women (20.5%), and the difference was statistically significant (p<0.05). A significant association for increasing infection rate with increasing age was observed (Table 4).
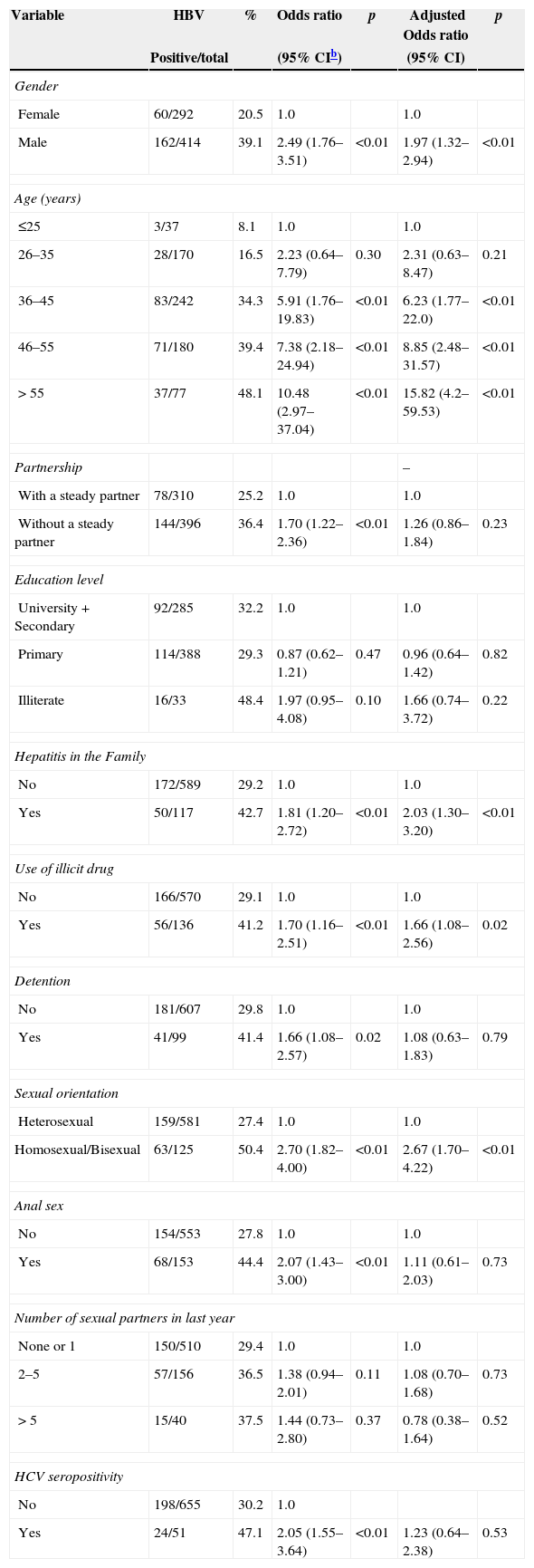
Univariate and multivariate analysis of factors associated with risk of acquiring infection with hepatitis B in HIV-infected patients in Campo Grande, central-West Region of Brazil, 2013 (n=706a).
| Variable | HBV | % | Odds ratio | p | Adjusted Odds ratio | p |
|---|---|---|---|---|---|---|
| Positive/total | (95% CIb) | (95% CI) | ||||
| Gender | ||||||
| Female | 60/292 | 20.5 | 1.0 | 1.0 | ||
| Male | 162/414 | 39.1 | 2.49 (1.76–3.51) | <0.01 | 1.97 (1.32–2.94) | <0.01 |
| Age (years) | ||||||
| ≤25 | 3/37 | 8.1 | 1.0 | 1.0 | ||
| 26–35 | 28/170 | 16.5 | 2.23 (0.64–7.79) | 0.30 | 2.31 (0.63–8.47) | 0.21 |
| 36–45 | 83/242 | 34.3 | 5.91 (1.76–19.83) | <0.01 | 6.23 (1.77–22.0) | <0.01 |
| 46–55 | 71/180 | 39.4 | 7.38 (2.18–24.94) | <0.01 | 8.85 (2.48–31.57) | <0.01 |
| > 55 | 37/77 | 48.1 | 10.48 (2.97–37.04) | <0.01 | 15.82 (4.2–59.53) | <0.01 |
| Partnership | – | |||||
| With a steady partner | 78/310 | 25.2 | 1.0 | 1.0 | ||
| Without a steady partner | 144/396 | 36.4 | 1.70 (1.22–2.36) | <0.01 | 1.26 (0.86–1.84) | 0.23 |
| Education level | ||||||
| University+Secondary | 92/285 | 32.2 | 1.0 | 1.0 | ||
| Primary | 114/388 | 29.3 | 0.87 (0.62–1.21) | 0.47 | 0.96 (0.64–1.42) | 0.82 |
| Illiterate | 16/33 | 48.4 | 1.97 (0.95–4.08) | 0.10 | 1.66 (0.74–3.72) | 0.22 |
| Hepatitis in the Family | ||||||
| No | 172/589 | 29.2 | 1.0 | 1.0 | ||
| Yes | 50/117 | 42.7 | 1.81 (1.20–2.72) | <0.01 | 2.03 (1.30–3.20) | <0.01 |
| Use of illicit drug | ||||||
| No | 166/570 | 29.1 | 1.0 | 1.0 | ||
| Yes | 56/136 | 41.2 | 1.70 (1.16–2.51) | <0.01 | 1.66 (1.08–2.56) | 0.02 |
| Detention | ||||||
| No | 181/607 | 29.8 | 1.0 | 1.0 | ||
| Yes | 41/99 | 41.4 | 1.66 (1.08–2.57) | 0.02 | 1.08 (0.63–1.83) | 0.79 |
| Sexual orientation | ||||||
| Heterosexual | 159/581 | 27.4 | 1.0 | 1.0 | ||
| Homosexual/Bisexual | 63/125 | 50.4 | 2.70 (1.82–4.00) | <0.01 | 2.67 (1.70–4.22) | <0.01 |
| Anal sex | ||||||
| No | 154/553 | 27.8 | 1.0 | 1.0 | ||
| Yes | 68/153 | 44.4 | 2.07 (1.43–3.00) | <0.01 | 1.11 (0.61–2.03) | 0.73 |
| Number of sexual partners in last year | ||||||
| None or 1 | 150/510 | 29.4 | 1.0 | 1.0 | ||
| 2–5 | 57/156 | 36.5 | 1.38 (0.94–2.01) | 0.11 | 1.08 (0.70–1.68) | 0.73 |
| > 5 | 15/40 | 37.5 | 1.44 (0.73–2.80) | 0.37 | 0.78 (0.38–1.64) | 0.52 |
| HCV seropositivity | ||||||
| No | 198/655 | 30.2 | 1.0 | |||
| Yes | 24/51 | 47.1 | 2.05 (1.55–3.64) | <0.01 | 1.23 (0.64–2.38) | 0.53 |
The univariate analysis showed associations between HBV infection with male gender, increasing age, having steady partnership, family history of hepatitis, use of illicit drug, previous incarceration, homosexual behavior, anal sex, and HCV infection. However, in the multivariate analysis, only male gender, increasing age, family history of hepatitis, use of illicit drug, and homosexual behavior remained significantly and independently associated with HBV infection in HIV positive patients (Table 4). Low education and income level, history of surgery and tattooing, shared personal objects, intravenous illicit drug use, condom use, multiple sex partners and history of blood transfusion were not statistically significant.
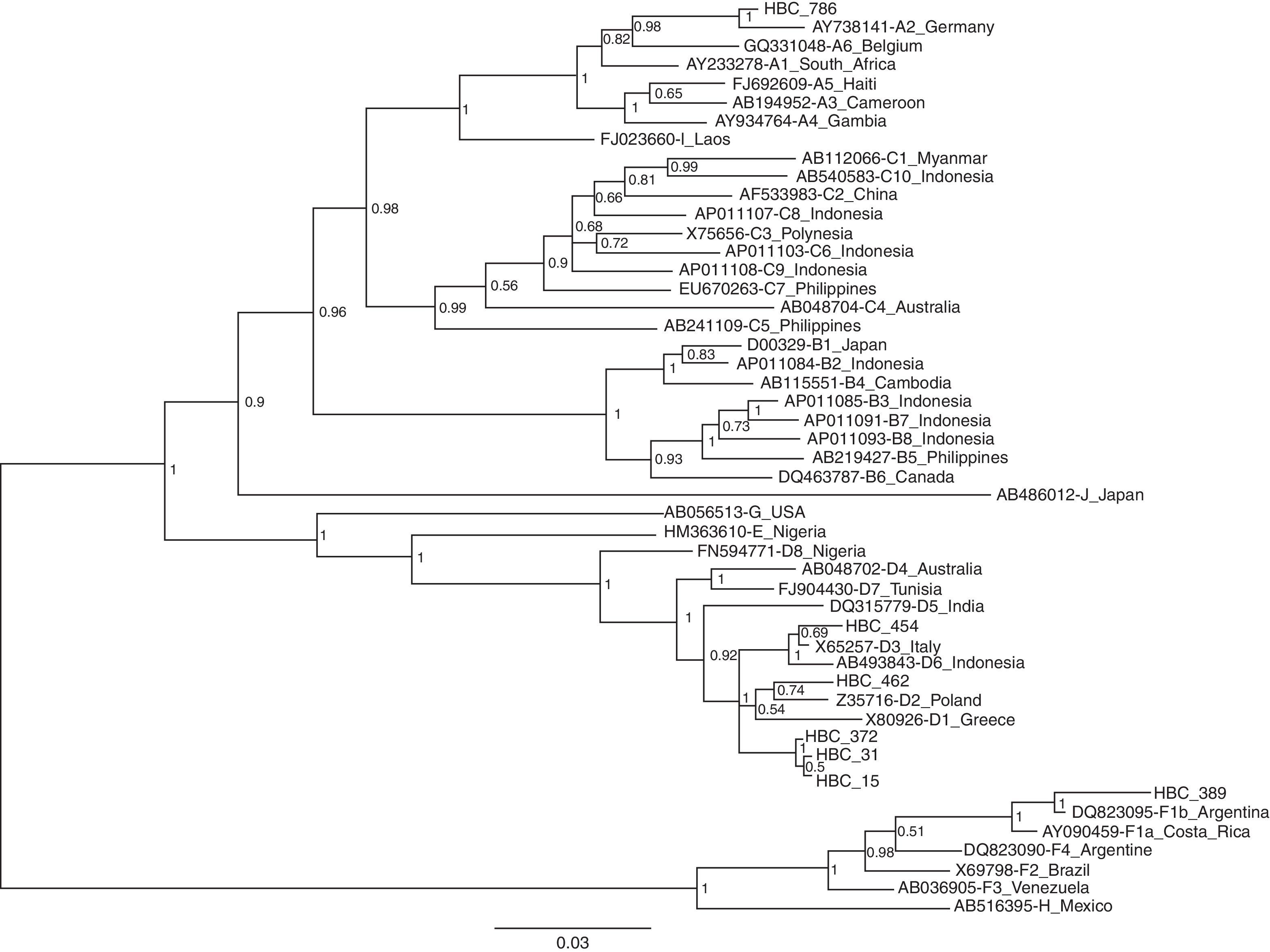
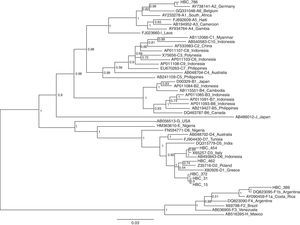
HBV-DNA was detected in 16 of the 21 HBsAg positive samples (76.2%) and 13 were successfully sequenced. The phylogenetic analysis based on pre-S/S region revealed that the distribution of HBV genotypes among these patients was A (n=1; 7.7%), D (n=10; 77.0%) and F (n=2; 15.3%). The genotype A isolated was from subgenotype A2. Among the 10 samples classified as genotype D, 6 were clustered in subgenotype D2 and one with D3. One of the 2 isolated F genotypes was subgenotype F2. The phylogenetic tree was constructed from 7 patients and reference sequences of HBV subgenotypes retrieved from GenBank. The evolutionary history was inferred using the Neighbor-Joining method. As seen in Fig. 1, each genotype clusters together.
Neighbor-joining phylogenetic tree based on pre S/S gene from 7 Brazilian HIV-HBV coinfected patients (HBC) and reference sequences representing all known HBV subgenotypes available at the GenBank. The numbers at the nodes represent the bootstrap support values (% obtained for 1000 replicates).
HIV and HBV have similar routes of transmission, and therefore, HIV positive individuals are at risk for coinfection with HBV. HIV infection leads to a significant change in epidemiological profile, natural history and treatment of chronic HBV infection resulting in higher rates of persistence and increasing liver-related morbidity and mortality.15
The prevalence of serological markers of HBV exposure in HIV-infected individuals varies according to the geographical area and the risk rates of the studied population. Knowledge on regional HBV prevalence is important for providers. The prevalence of HBV infection among HIV-infected patients measured in this study (26.2%) was lower than those reported in sub-Saharan Africa (59.8%),16 Chile (46.3%),17 Cuba (45.5%)18 and in different regions of Brazil, which ranges from 30.0% to 55.1%.4,5,7,19,20
The prevalence of HIV-HBV coinfection (2.5%) in the present study was lower than those reported in different regions of Brazil (5.7%, 8.5%, and 18%).4,5,21 In addition, our results were in line with the prevalence of HIV-HBV coinfection from the state of Mato Grosso, also located in Central-West Region of Brazil.20
In the population-based multicentric survey of hepatitis B infection conducted in urban area of Central-West Region of Brazil, the region was classified as a low HBV endemic area based on the encountered prevalence.22 On the other hand, remote sites highly endemic for HBV have been reported in the Afro-descendant communities in Central-West, even in Mato Grosso do Sul state.23,24 Therefore, this low HIV-HBV coinfection rate could be attributable to the low HBV infection endemicity found in the urban area of Central-West Region of Brazil.
Therefore, HIV-infected patients with isolated anti-HBc should be vaccinated against HBV.25,26 The inability to detect HBsAg in individuals positive for anti-HBc alone can be attributed to different factors such as low level of HBV replication, HBV genotype variations and mutations in pre-S/S genomic region, which can affect HBsAg detection by conventional techniques.27
The vaccination against hepatitis B infection of all people living with HIV, with post-vaccination testing and revaccination of full series non-responders, is a cornerstone of HBV prevention.28,29 Only 16.7% of 848 HIV infected participants had protective anti-HBs titers. In our region, immunization against hepatitis B was not routinely offered for all HIV-infected individuals to prevent primary HBV infection. The lack of response to hepatitis B vaccination among HIV-infected patients may be because they were not vaccinated or due to immunologic impairment resulting in decreased response to the vaccine. Therefore, providing higher and additional doses of vaccine or using immunomodulatory agents are methods to enhance the immune response in non-responders.28 Also, a large number of patients (57.1%) are still susceptible to HBV infection. Despite immunization against hepatitis B not being routinely offered for all HIV-infected individuals to prevent primary HBV infection, these finding underscore the need to ensure early vaccination as a means of primary prevention against HBV infection.
Few studies on HIV-infected patients with multiple hepatitis viruses have been published to date.30 No HIV-HBV patients had anti-HDV positivity, confirming that HDV infection is infrequent in the Central-West Region of Brazil.20 The prevalence of HIV/HBV/HCV in HIV-infected patients is below 3%, but much higher than that in the general population.26 In our study, the prevalence of HIV/HBV/HCV coinfection (0.1%) is similar to other rates previously reported (0.1% and 0.3%).31,32
After multivariate analysis, serological evidence of HBV infection was significantly associated with male gender, age over 35 years, family history of hepatitis, use of illicit drug and homosexual behavior. A predominance of the male gender has also been reported previously in other studies possibly due to high-risk behaviors for HBV infection increasing sexual and percutaneous exposure.17,19,33 This trend may be explained on the basis of the higher rate of injecting drug users (IDU) among the males studied (86.6% – data not showed).
Significant increase in the seroprevalence of HBV was observed among older adults. This finding was in line with previous studies, which reported that older age was associated with a higher risk of HBV exposure.18,19,25 History of hepatitis in the family was also strongly associated with serological markers of previous exposure to HBV, and probably indicates that most HBV exposure is associated with the patients’ close contacts (e.g. family environment).32
This study also found that the non-intravenous illicit drug abuse remained significantly and independently associated with HBV coinfection. Injection and non-injection drug use are identified as important factors associated with unsafe sexual practices, putting illicit drug users at high risk for HBV infection and transmission.19,34
A strong association between homosexual activity and HBV coinfection was also observed, which is in agreement with previous reports and demonstrates that HBV infection is highly linked to sexual intercourse, including men who have sex with men (MSM).4,18,35 Although the majority of HIV-positive Brazilians become infected through heterosexual sex, MSM still face a proportionally higher risk. Studies conducted in 10 Brazilian municipalities between 2008 and 2009 estimated MSM are around 11 times more likely to become infected with HIV than heterosexual individuals, with HIV prevalence rates of 12.6% among MSM.36 MSM should be encouraged to use condoms during anal sex in order to reduce the risk of HIV-HBV coinfection.
The molecular characterization of the HBV sequences is also important to establish evolutionary origins and patterns of viral dispersal.37 Among HBsAg-positive patients, HBV genotypes D, F and A were found. This study supports previous findings that confirm the predominant movement of these genotypes in the Brazilian population, as well as in our region.37–39
The present study has certain limitations. As the study design was cross-sectional, a causal relationship between the time of exposure and subsequent infection could not be established. However, the study was conducted in two main reference centers of infectious diseases where nearly all of the HIV-infected patients are diagnosed and followed-up in the state of Mato Grosso do Sul, Central-West Region of Brazil.
These results suggest that people infected with HIV are exposed to HBV at a higher rate than the general population and the prevalence of HIV-HBV coinfection in our region is lower than that in other regions of Brazil. Male gender, increasing age, family history of hepatitis, use of illicit drug and homosexual contact play an important role in the transmission of these two viruses. Furthermore, detecting and immunizing susceptible individuals and revaccination of HIV-infected individuals who did not respond to the standard HBV vaccination schedule is an important strategy for lowering the incidence of HBV infection among HIV-infected individuals. Further investigations at clinical, molecular and immunological levels to elucidate the interaction of HBV-HIV coinfection among these patients are warranted.
Conflicts of interestThe authors declare no conflicts of interest.
The authors would like to thank all the participants and the staff of the Esterina Corsini University Hospital and from the Reference Center of Infectious and Parasitic Diseases (CEDIP) for questionnaire data and blood sample collection.