Molecular tests allow the detection of high-risk human papillomavirus in cervical samples, playing an important role in the prevention of cervical cancer.
ObjectivesWe performed a study to determine the prevalence of HPV 16, HPV 18 and other high-risk human papillomavirus (pool 12 genotypes) in Peruvian females from diverse urban areas using the cobas 4800 HPV test.
MethodsRoutine cervical samples collected in our laboratory were analyzed by cobas 4800 HPV test.
ResultsA total of 2247 samples from female patients aged 17–79 years were tested. high-risk human papillomavirus was positive in 775 (34.49%) samples. Of these, 641 (82.71%) were single infections and 134 (17.29%) were multiple infections. The positivity rates for HPV 16, HPV 18, and other high-risk human papillomavirus were 10.77%, 2.0%, and 28.08%, respectively. In multiple high-risk human papillomavirus infections, the concomitance of HPV 16 and other high-risk human papillomavirus was more prevalent (13.42%).
ConclusionOur study showed high prevalence of high-risk human papillomavirus in urban Peru, mainly among young women. In both single and multiple infections other high-risk human papillomavirus were more prevalent than HPV 16 and HPV 18, which might influence vaccine impact in our country. Furthermore, the cobas 4800 HPV test may be considered a useful tool for HPV molecular diagnosis.
Cervical cancer is the second most common cause of cancer in women1 and it is estimated that each year approximately 493,000 new cases are diagnosed and 274,000 women die from cervical cancer worldwide.2 Human Papillomavirus (HPV) has been recognized as an important cause of cervical cancer3 and is implicated in 99.7% of cervical squamous cell cancer cases in the world.4 More than 40 HPV genotypes have been detected in the anogenital mucosa and are usually transmitted through sexual activity.5 The HPV genotypes 16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59, 66, and 68 are associated with cervical cancer and have been classified as high-risk (HR) group.6,7
A global meta-analysis showed that the prevalence of HPV in 157,879 women with normal cytology was 10.4%. The prevalence estimates by region were Africa 22.1%, Central America and Mexico 20.4%, Northern America 11.3%, Europe 8.1%, and Asia 8.0%.8 Of all high-risk human papillomavirus (HR-HPV) genotypes, HPV 16 has been regarded as the most prevalent genotypes.9
Few studies have examined the epidemiology of HPV in Peru. In the Department of San Martin, 5435 women were tested for HPV by Hybrid Capture II (Qiagen, Gaithersburg, MD); the prevalence of HPV was estimated to be 12.6%.10 On the other hand, the overall presence of HR-HPV was 33.6% in a study of 2208 women with abnormal cytology from different cities in Peru.11 Also, an analysis performed on 384 cervical samples from six regions with the highest percentage of rural inhabitants in Peru showed the presence of HPV in 73 cases (19.01%) and the most common genotype was HPV 16, found in six (8.22%) cases.12
The DNA testing of HR-HPV genotypes in cervical samples has a sensitivity of up to 96.6% for detecting precancerous lesions.13 The cobas 4800 HPV Test (Roche Molecular Systems, Inc) is a qualitative in vitro test for the detection of HPV in patient specimens. The test utilizes amplification of target DNA by real time PCR and nucleic acid hybridization for the detection of 14 HR-HPV genotypes in a single analysis. The test specifically identifies genotypes 16 and 18 while simultaneously detecting the other HR genotypes (pool of 12 genotypes: 31, 33, 35, 39, 45, 51, 52, 56, 58, 59, 66, and 68). The ATHENA (Addressing THE Need for Advanced HPV Diagnostics) study, involving more than 47,000 women in the United States, demonstrated that the cobas 4800 HPV Test is clinically validated for ASCUS triage.14
The present study aims to determine the prevalence of HPV 16, HPV 18 and other HR-HPV (pool of 12 genotypes) in cervical scrapes from women living in diverse urban areas in Peru by the cobas 4800 HPV Test.
Materials and methodsStudy populationDuring the period from November 2011 to October 2013, routine cervical samples collected from women living in different urban areas across the country were received at the laboratory. Urban areas include the cities of Iquitos, Cajamarca, Piura, Chiclayo, Lima, Arequipa, Cuzco and Juliaca. On date of arrival, the samples were registered and labeled with a barcode for personal identification.
Cobas 4800 HPV testThe cobas 4800 HPV test is a qualitative assay for the presence of HPV 16, HPV 18 and a pool of 12 other HR-HPV genotypes (31, 33, 35, 39, 45, 51, 52, 56, 58, 59, 66 and 68). This test requires 1mL of liquid cytology sample. No pre-treatment of samples is required. The cobas 4800 HPV test was performed according to the recommendations of the manufacturer.15 Briefly, DNA was extracted, purified and prepared for PCR by the cobas x 480 instrument. Amplification and detection of HR-HPV DNA were undertaken on the cobas z 480 analyzer. Fluorescent TaqMan probes were used for detection of the amplicons during PCR cycles.
Both positive and negative control specimens are included in each run. In addition, the test detects beta-globin DNA as an internal control of sufficient specimen cellularity. Specialized pipetting technology combined with AmpErase enzymes reduces cross contamination risk.
AnalysisHPV detection in cervical samples was considered as positive or negative. Prevalence of HPV including HPV 16, HPV 18 and other HR-HPV (pool of 12 genotypes) was reported. The frequencies of single and multiple HPV infections were also described. The results of the cobas testing were tabulated by age of the female patients.
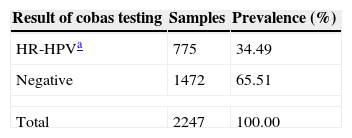
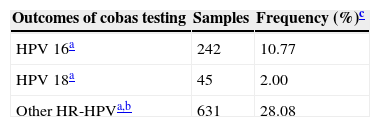
ResultsA total of 2247 cervical samples were analyzed. Patients were with the age 17–79 years (average 34.48 years). All cervical samples contained sufficient DNA to perform PCR, based on amplification of the human beta-globin gene. In the present study, HR-HPV was detected in 775 samples and the overall prevalence was 34.49% (Table 1). HPV 16 was detected in 242 samples, HPV 18 in 45 samples, and other HR-HPV (pool of 12 genotypes) in 631 samples, either as single or multiple infections combined (Table 2). The prevalence of other HR-HPV (28.08%) was higher than HPV 16 (10.77%) and HPV 18 (2.0%).
Prevalence of HR-HPV among 2247 samples.
| Result of cobas testing | Samples | Prevalence (%) |
|---|---|---|
| HR-HPVa | 775 | 34.49 |
| Negative | 1472 | 65.51 |
| Total | 2247 | 100.00 |
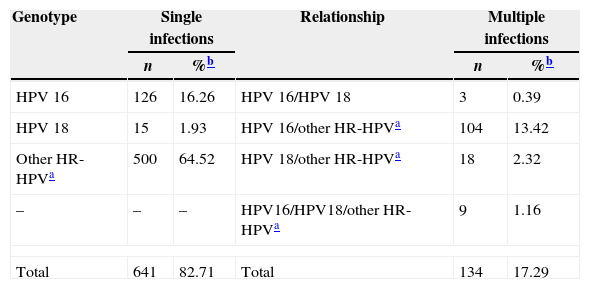
Of 775 HPV positive samples, 641 (82.71%) were single infections and 134 (17.29%) were multiple infections. Other HR-HPV (pool of 12 genotypes) were the most common in single (500/641) and in multiple infections (131/134). In single infections, HPV 16 was detected in 126 samples, whereas HPV 18 in 15. With respect to multiple HPV infections, HPV 16 and other HR-HPV were the most common (13.42%) coinfections, while HPV 16 and HPV 18 coinfections were the least common (0.39%). Also we observed the simultaneous presence of HPV 16, HPV 18 and other HR-HPV at least in nine samples (Table 3).
Single or multiple infections in 775 HPV positive samples.
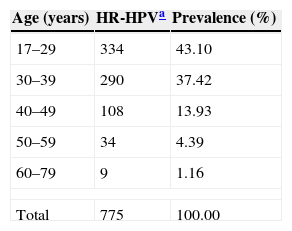
The prevalence of HR-HPV was highest in younger women and showed a decreasing trend with age (Table 4). The prevalence according to age was as follows: 43.1% for women under 29 years; 37.42% for those between 30 and 39 years; 13.94% in the age range 40–49; 4.39% between 50 and 59; and 1.16% among those aged 60–79.
HR-HPV detection according to age.
| Age (years) | HR-HPVa | Prevalence (%) |
|---|---|---|
| 17–29 | 334 | 43.10 |
| 30–39 | 290 | 37.42 |
| 40–49 | 108 | 13.93 |
| 50–59 | 34 | 4.39 |
| 60–79 | 9 | 1.16 |
| Total | 775 | 100.00 |
The high prevalence of HR-HPV (34.49%) in women from different urban areas of Peru is similar to that described in other countries such as Russia, 29.1%; Trinidad and Tobago, 35.4%; and East African countries, 33.6%.8,16 But it was higher in other parts of Latin America, such as Mexico, 14.5%; Costa Rica, 16.0%; Colombia, 20.5%; and Ecuador, 24.2%.17–20 However, it was lower than the rate found in Brazil (Rio de Janeiro).21 As it happens in most developing countries, some socio-economic and cultural factors may be related to this high HPV prevalence, especially early start of sexual activity; multiparity; lack of education; and the misconceptions and beliefs that constrain people from discussing diseases of the genital tract.22
In Peru, previous studies found that the prevalence of HPV in the Department of San Martin was 12.6%10 and in some rural areas was 19.01%,12 which were lower than the prevalence obtained in the present study. However, our data are consistent with those obtained by Li Ning et al.,11 where the prevalence of HR-HPV in different cities of our country was 33.6%.
This is the first study about prevalence of HR-HPV using the cobas 4800 HPV Test in our country. As a part of our routine, cervical samples were received for HPV molecular diagnosis. The test identifies the presence of HPV 16, HPV 18 and other HR-HPV (pool of 12 genotypes), which are involved with cervical cancer.23 In both single and multiple infections, other HR-HPV were more common than HPV 16 and HPV 18, similar to a previous study in 5072 women from Denmark.24 This is because infections by other HPV genotypes (not 16/18) are more frequent in women with normal cytology or low-grade CIN, although these genotypes only cause about one-third of cervical cancer.25 Furthermore, our results are in line with studies indicating higher prevalence of HR-HPV in young women (17–29 years) and HPV infection decreasing with aging24,26,27 However, it is important to identify which are the most common genotypes in the group of other HR-HPV. This would help to include the prevailing genotypes in the design of multivalent vaccines. It is also desirable to incorporate these genotypes in the next generation of automated molecular diagnostic systems.
Several studies assessed the use of the cobas 4800 HPV test.24,28–31 Those studies showed that the test have a high specificity and sensitivity, less cross contamination risk, clinical relevance, sufficient interlaboratory reproducibility, and less labor intensive. Additionally, this test fulfills all requirements as defined by the international guidelines32 when assessing a test for screening purposes.
In conclusion, our study showed a high prevalence of HR-HPV in females from diverse urban areas of Peru especially among young women. Both single and multiple HR-HPV infections underscore the presence of HR-HPV (pool of 12 genotypes) in excess of HPV 16 and HPV 18. This finding might influence vaccine impact in our country. Finally, the cobas 4800 HPV Test proved to be a useful tool for HPV molecular diagnosis.
Conflicts of interestThe authors declare no conflicts of interest.