Zika virus (ZIKV) is an emergent flavivirus transmitted mainly through Aedes spp. mosquitoes that is posing challenge to healthcare services in countries experiencing an outbreak. Usually ZIKV infection is mild, but in some cases it has been reported to progress into neurological diseases such as microcephaly in infants and Guillain-Barré syndrome (GBS) in adults. GBS is a debilitating autoimmune disorder that affects peripheral nerves. Since ZIKV caused massive outbreaks in South America in the past few years, we aimed to systematically review the literature and perform a meta-analysis to estimate the prevalence of GBS among ZIKV-infected individuals. We searched PubMed and Cochrane databases and selected three studies for a meta-analysis. We estimated the prevalence of ZIKV-associated GBS to be 1.23% (95% CI=1.17–1.29%). Limitations include paucity of data regarding previous flavivirus infections and ZIKV-infection confirmation issues. Our estimate seems to be low, but cannot be ignored, since ZIKV outbreaks affects an overwhelming number of individuals and GBS is a life-threatening debilitating condition, especially in pregnant women. ZIKV infection cases must be closely followed to assure prompt care to reduce the impact of GBS associated-sequelae on the quality of life of those affected.
Zika virus (ZIKV) is an arbovirus that belongs to the Flaviviridae family, the same of Dengue (DENV) virus.1 ZIKV is mainly transmitted through Aedes spp. mosquitoes’ bites, but transmission by blood transfusion and sexual intercourse can also occur.2 Non-human primates seem to be the reservoir hosts for ZIKV, but the primary species have not yet been identified.3
Until 2007, ZIKV was limited to Africa and Asia, and only mild cases were reported.4 After 2007, ZIKV cases started to increase remarkably, causing outbreaks in many different countries. The first major outbreak of ZIKV infection was recorded in French Polynesia in October 2013.2 The virus subsequently spread through the Pacific reaching Brazil in mid-2015; the largest outbreak was reported in this country, and up to date the World Health Organization (WHO) has estimated approximately 3–4 million cases of ZIKV.3
ZIKV infection is thought to be symptomatic only between 18% and 35% of the cases, resulting in a mild illness with symptoms such as fever, myalgia, maculopapular rash, and arthralgia.3,5,6 However, there is now growing evidence that ZIKV infection is related to a range of neurologic disorders, such as Guillain-Barré syndrome (GBS) in adults7 and microcephaly in infants.2 This link has been hypothesized after the parallel upsurge of Zika cases along cases of GBS and microcephaly, particularly during the Brazilian outbreak. Consequently, the increase in the number of cases led the WHO to declare a public health emergency of international concern. However, it is relevant to mention that some cases of ZIKV have been probably misclassified because of the molecular similarity of the virus with DENV1 leading to serological cross reactivity in serologic tests employing antibodies for laboratory diagnosis; the RT-PCR based molecular test is more effective but only useful during the first week of infection.
GBS is a rare but serious autoimmune syndrome that attacks the peripheral nerves, causing a progressive paralysis. Typically, an infection eventually triggers autoantibodies targeting gangliosides in the membrane of nervous cells, causing respiratory or gastrointestinal symptoms before more obvious neurologic/motor impairment.8 It has a mortality rate of approximately 5%, and 20% of the patients affected usually are left with significant disability.3 This syndrome was already associated with ZIKV infection during the outbreak in French Polynesia. Indeed, the rise and fall of Zika cases was followed by a similar trend in the onset of GBS.4 However, the mechanisms by which ZIKV infection causes GBS have not yet been established; it has been suggested that the virus could exacerbate the immune response triggering an immunopathogenic process that determines, in turn, the onset of GBS.3 Some patients “developed neurological symptoms during or immediately after the [ZIKV] infection, suggesting a parainfectious rather than postinfectious pattern that is typically seen in GBS”, as noted in a recent review.9 These symptoms include muscle weakness, inability to walk, facial palsy, and respiratory distress. In 2015, 1708 cases of GBS were reported in Brazil representing a 19% increase in comparison to the previous year.2
Therefore, we aimed to systematically review the literature and perform a meta-analysis to estimate the prevalence of GBS among ZIKV-infected individuals and discuss the most recent findings regarding the relation between ZIKV and GBS.
MethodsLiterature search and systematic reviewFollowing the recommendations of the PRISMA statement for conducting systematic reviews,10 we searched for studies published on international journals regarding ZIKV infection and its relationship with GBS. Our inclusion criteria were epidemiological/observational studies, such as case series, epidemiological surveys, cross-sectional or cohort studies, which should have involved adult individuals. Exclusion criteria were review studies, studies written in languages other than English, Portuguese or Spanish and papers without explicit numerical data needed for the calculation of the meta-analysis.
The used keywords were “Zika” and “neurological defects”. We searched for papers published anytime until early November 2017 in two databases: PubMed and Cochrane Library. On the PubMed database, 53 abstracts were found. On the Cochrane Library database, the keyword used was only “Zika”, retrieving 23 papers, since the combination with “neurological defects” did not result in any paper.
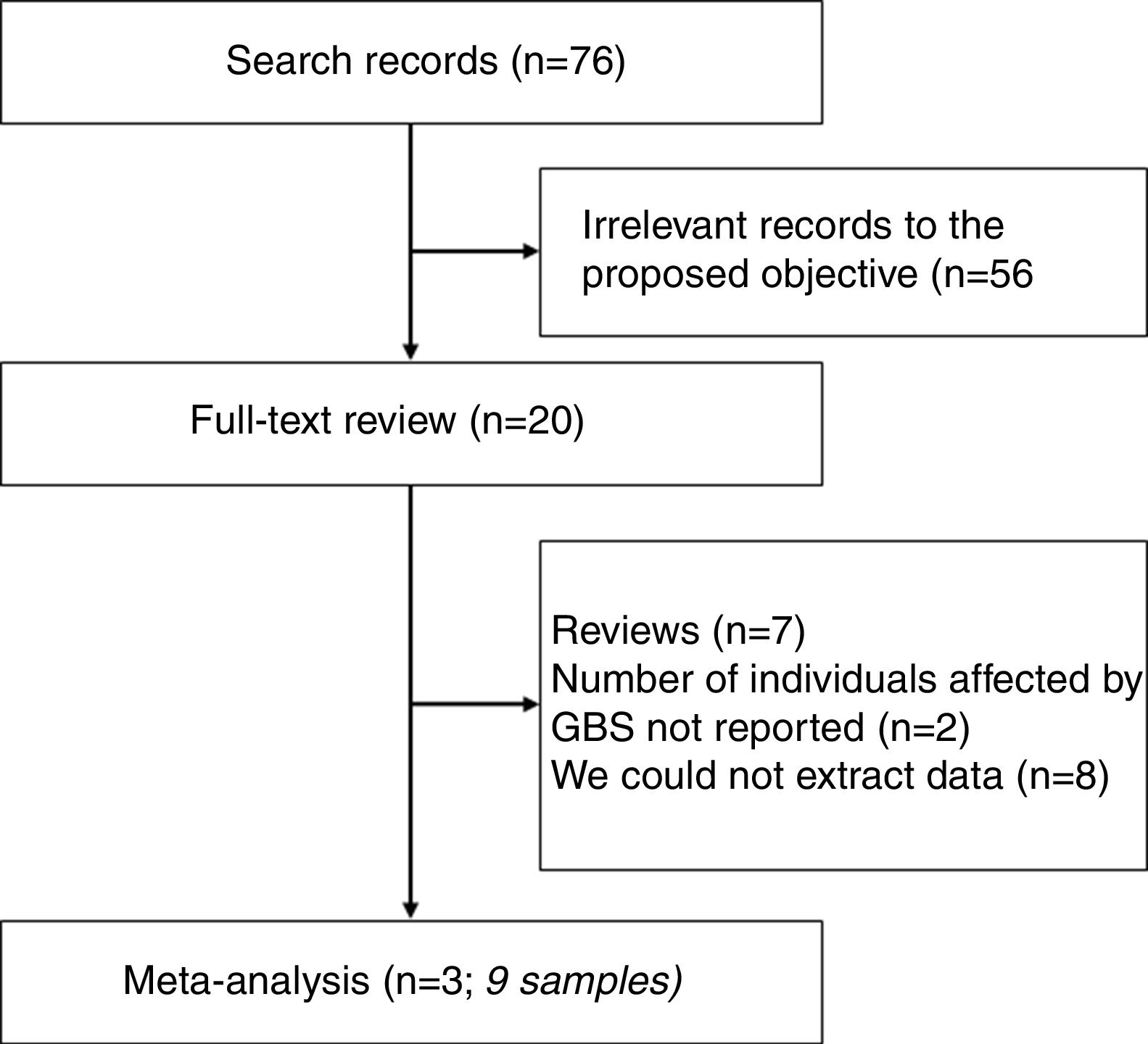
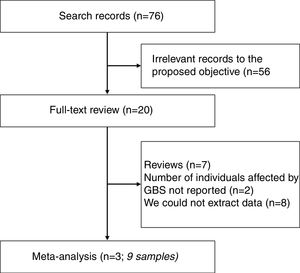
Thus, the initial systematic review strategy found 76 abstracts. Among these, 56 abstracts were not relevant to our objective. The remaining 20 studies full-text were further reviewed. Among those, seven were review studies, two did not report number of individuals affected by GBS, and we could not extract the necessary data from eight studies. Therefore, three studies (totaling nine samples) were selected for further meta-analysis.11–13
Two of the authors performed data collection independently and registered studies’ data in electronic spreadsheets. Additionally, each author critically reviewed the quality of the studies by answering a CASP checklist14 and the studies were deemed to have satisfactory quality.
The authors unified the data, resolving any inconsistencies or omissions before further analysis. The data collected from the studies included year of sampling/publication, country where the study was performed, and sample sizes (number of ZIKV infection cases and number of GBS cases). These data were used to calculate the outcome of interest, which was the observed GBS prevalence among ZIKV-infected individuals (in other words, the number of GBS cases divided by the number of ZIKV infected cases). The individual observed GBS prevalence were then included in a meta-analysis for the calculation of a pooled ZIKV infection-associated GBS prevalence.
Meta-analysisThe GBS prevalence estimates (proportions) were log-transformed for the pooled estimation through inverse-variance weighting method15 and back transformed to simple proportions alongside 95% confidence intervals (95% CI). The meta-analysis was conducted with a fixed effect model assumption.
The heterogeneity between studies’ sample sizes was expressed through I2 measure and τ2 statistic. A Cochran's Q test with n−1 degrees of freedom (in which n is the number of studies included, and with significance level α=0.10 for this test) was conducted to assess if heterogeneity was significantly different from zero. If so, the heterogeneity was classified according to the observed I2 measure: ≤25%, between 25 and 50%, between 50 and 75%, and between 75 and 100%, respectively considered as low, moderate, high, and very high heterogeneity.
The meta-analysis was performed through the “meta” package16 for R software, version 3.4.1 (R Core Team, Vienna, Austria) (June 2017).17
ResultsSummary of the selected studiesAs mentioned before, three studies were selected for inclusion in a meta-analysis.11–13Fig. 1 is a flowchart showing studies selection. At the time of reporting of the first study (February 2014), which was conducted in French Polynesia, DENV seropositivity was around 23.2%, with an estimated number of 12,400–25,700 people infected by DENV during the 2013–2014 French Polynesian outbreak. The estimated number of ZIKV infected individuals during this outbreak was 29,000 individuals. However, just 8262 suspected cases of ZIKV infection were reported, since most infected individuals did not search healthcare and the diagnosis was not laboratory confirmed; among these, 396 cases were confirmed by RT-PCR. Out of the 38 French Polynesian GBS-affected patients, 16 needed intensive care unit hospitalization. At the time of reporting, five were still hospitalized. No GBS-associated deaths occurred.11
The second study was conducted in the Netherlands and was an observational study that conducted a follow-up of 18 ZIKV infected adult Dutch individuals (mean age 49 years, range 8 to 61 years; 12 females) who had traveled to Suriname and Dominican Republic. A single case of GBS was observed, in a 60-year-old woman. The authors reported that she had an “underlying illness”, but it was not clear what this condition was, and they did not elaborate further if it was preexistent or could have predisposed to GBS occurrence. Dengue virus-specific immunoglobulin G (IgG) positive serology was present in four patients and weak IgM positive serology was present in three individuals out of 13 with available data among the 18 Dutch individuals; eight of them had previously received yellow fever vaccination.12
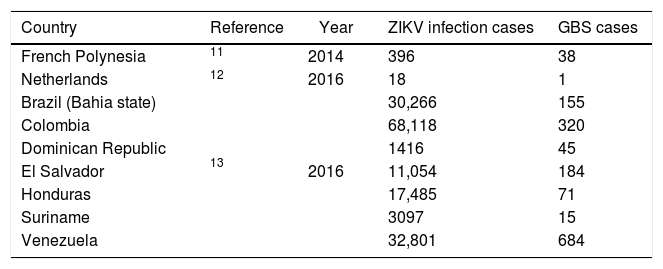
The third study summarized case series from seven South American, Central American and Caribbean countries. The authors analyzed health reports and observed an aggregated number of 164,237 cases of ZIKV infection reported between January 2015 and March 2016 in Brazil (state of Bahia), Colombia, Dominican Republic, El Salvador, Honduras, Suriname, and Venezuela. Among these cases, 1474 cases of GBS were reported.13
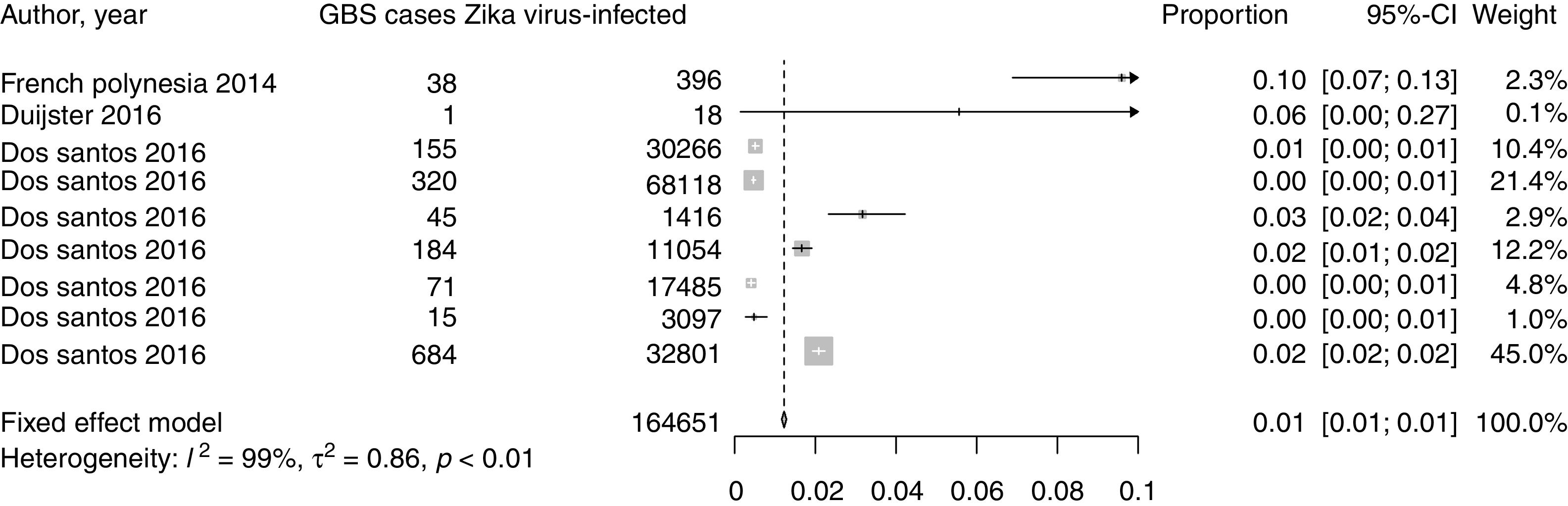
Meta-analysis resultsThe total sample number polled by the meta-analysis was 164,651 ZIKV-infected individuals. Among them, 1513 developed GBS. According to a fixed effect meta-analysis model, an estimated 1.23% of ZIKV infection cases could progress to GBS (95% CI=1.17–1.29%). A very high sample size heterogeneity was observed, I2=99.1% and τ2=0.86 (Cochran's Q=932.3 with eight degrees of freedom, p<0.001).
These results are summarized in Fig. 2, which is a Forest plot displaying the studies, their sample sizes and confidence intervals, and in Table 1, which lists the characteristics of the studies included in the meta-analysis.
Forest plot of the prevalence of GBS associated with ZIKV infection. The plot displays pooled sample size (164,651 ZIKV-infected individuals), individual prevalence estimates by each study, pooled prevalence estimate (fixed effects model), the corresponding 95% confidence intervals, study weighting (fixed effects model) and sample size heterogeneity measures and Cochran's Q test for heterogeneity p-value.
Summary of studies’ characteristics.
| Country | Reference | Year | ZIKV infection cases | GBS cases |
|---|---|---|---|---|
| French Polynesia | 11 | 2014 | 396 | 38 |
| Netherlands | 12 | 2016 | 18 | 1 |
| Brazil (Bahia state) | 13 | 2016 | 30,266 | 155 |
| Colombia | 68,118 | 320 | ||
| Dominican Republic | 1416 | 45 | ||
| El Salvador | 11,054 | 184 | ||
| Honduras | 17,485 | 71 | ||
| Suriname | 3097 | 15 | ||
| Venezuela | 32,801 | 684 |
ZIKV, Zika virus.
GBS, Guillain-Barré syndrome.
ZIKV infection usually has a mild presentation, but recently it has been demonstrated its relationship with central nervous system sequelae, such as microcephaly in newborns, encephalitis, and GBS, which are being regarded as the “neurological syndrome of ZIKV infection”.18 Therefore, any ZIKV suspected infection must be closely followed,19 especially in pregnant women, since several studies indicate that ZIKV impairs central nervous system development of fetuses.20 Moreover, whereas GBS mortality occurs in around 5% of the cases, this number rises to 10–35% if the GBS-affected person is a pregnant woman.8
Despite the growing evidence, currently there are no results from prospective cohort studies about ZIKV neurological consequences. Therefore, we performed a systematic review of studies and conducted meta-analysis modeling to estimate the prevalence of GBS associated with ZIKV infection.
We estimated the prevalence of GBS to be 1.23% of all ZIKV infection cases in adults. This small number is deceptive, because GBS occurrence in the general population is rare, but when considering ZIKV infected cohorts, the incidence is several times larger. As remarked in a previous publication,21 ZIKV can infect a substantial number of individuals, which brings a new perspective to these seemingly small numbers. To illustrate this, we highlight the findings of a case series (included in our meta-analysis) with data from seven countries of South and Central America in 2015 and early 2016. In El Salvador, the number of GBS diagnosis was 100% (two times) higher than pre-ZIKV infection outbreak. In Venezuela, this increase was an impressive 877% (almost 10 times higher). In the state of Bahia, Brazil, one of the most affected countries by the epidemics, the number of GBS diagnosis almost tripled during the same period (increase of 171%).13 Therefore, if new outbreaks of ZIKV are expected, then the occurrence of GBS cases will likewise accompany them, bringing new burden to individuals and healthcare services in the affected countries.
Our meta-analysis has some limitations. First, we could not assess if the individuals had previous flavivirus infections (such as DENV or yellow fever virus), because suitable clinical and/or laboratory data were lacking. GBS in association with DENV and other flavivirus has also been reported,9 but it is currently unknown if previous infections could increase the risk for ZIKV-associated GBS occurrence due to cross-reactivity. More research is needed to address this issue. Second, some of the reported ZIKV infection are just suspected (not confirmed by RT-PCR or other methods).13 Besides these two limitations, it is important to remark that symptomatic ZIKV infection are the minority among the cases.3,5,6 Therefore, our ZIKV infection-associated GBS prevalence estimate may be underestimated, in theory.
ConclusionWe performed a meta-analysis to obtain a prevalence estimate of GBS caused by ZIKV infection. This estimate seems to be low, but cannot be ignored, since ZIKV outbreaks affects an overwhelming number of individuals, mostly in South and Central America, posing a challenge to healthcare services. ZIKV infection cases must be closely followed to assure prompt care to reduce the impact of GBS associated-sequelae on the quality of life of those affected.
FundingThis work was supported by grants 88881.130808/2016.1 from Comissão de Aperfeiçoamento de Pessoal do Nível Superior (CAPES) and 440371/2016-3 from Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq), from the Brazilian public notice Chamada 14/2016 – Prevenção e Combate ao vírus Zika, both granted to SC. AVCC was supported by stipends by CAPES (88887.137992/2017-00) from the same public notice and by Fundação de Amparo à Ciência e Tecnologia de Pernambuco (FACEPE – BFP-0018-2.02/17).
Conflicts of interestThe authors declare no conflicts of interest.