Carbapenem-Resistant Gram-Negative (CRGN) Bloodstream Infections (BSI) represent a therapeutic challenge, especially in the context of Febrile Neutropenia (FN) in cancer patients.
MethodsWe characterized pathogens causing BSI in patients aged ≥18 years who had undergone systemic chemotherapy for solid or hematological cancers between 2012 and 2021 in Porto Alegre, Brazil. Predictors of CRGN were evaluated through a case-control analysis. Each case was matched to two controls from whom CRGN were not isolated and had the same sex and year of inclusion in the study.
ResultsFrom 6094 blood cultures evaluated, 1512 (24.8%) showed positive results. Gram-negative bacteria accounted for 537 (35.5%) of the isolated bacteria, of which 93 (17.3%) were carbapenem-resistant. From 105 patients included in the case-control analysis, all cases had baseline hematological malignancies (60% acute myeloid leukemia). Variables related to CRGN BSI in Cox regression analysis were the first chemotherapy session (p<0.01), chemotherapy performed in the hospital setting (p = 0.03), intensive care unit admission (p<0.01), and CRGN isolation in the previous year (p<0.01). Patients with CRGN BSI received 75% less empirical active antibiotics and had 27.2% higher 30-day mortality rates than controls.
ConclusionsA CRGN risk-guided approach should be considered for empirical antibiotic therapy in patients with FN.
In recent years, the prognosis of patients with oncological diseases has significantly improved with the development of new therapeutic options. Despite the advances, chemotherapy remains essential for cancer treatment, which may increase the number of patients with neutropenia and severe infections.1 Infection-related mortality remains high in patients with Febrile Neutropenia (FN), accounting for up to 55% of all cases.2 Bacterial infections are most prevalent in this scenario, with Gram-Negative Bacteria (GNB) accounting for a high proportion of cases.3,4 Antibiotic resistance among GNB, especially carbapenem resistance due to carbapenemase production, such as Klebsiella pneumoniae Carbapenemase (KPC) or New-Delhi Metallo-beta-lactamase (NDM), is currently a major therapeutic challenge.5
The indications for empirical antibiotic coverage for Carbapenem-Resistant Gram-Negative (CRGN) bacteria in patients with FN are not well defined and should be based on local epidemiology and individual risk factors.6 Therapeutic options for these infections usually rely on drugs such as new beta-lactam/beta-lactamase inhibitors with activity against Class A-producing carbapenemase bacteria. However, some of these antibiotics are either unavailable, or their use is cost-prohibitive in low-income and developing countries. Old drugs, such as polymyxins and aminoglycosides, can be used; however, they are highly toxic. The benefit of empirically covering carbapenem-resistant GNB during FN should outweigh the risk of increasing the selective pressure for bacterial resistance. Therefore, physicians must judiciously and appropriately use these last-line antimicrobials.5 The main challenge is accurately identifying patients at high risk for these infections and the timely institution of adequate antimicrobial therapy.
Identifying pathogens causing Boodstream Infection (BSI) in patients undergoing chemotherapy and determining the risk factors for infections by CRGN bacteria is essential to understand which patients would benefit from early initiation of antibiotics with activity against these organisms. This would allow adjusting empirical treatment protocols based on risk assessment during an FN episode.
Our primary objective was to describe the epidemiology of BSI in patients with cancer and assess the risk factors for infections by CRGN bacteria in patients diagnosed with FN. The secondary outcomes measured were adequacy of empirical antimicrobial therapy, 30-day mortality, and in-hospital mortality.
This research complied with the recommendations of Resolution nº 466/12 and 510/16 of the National Health Council for Scientific Research in Human Beings. The study was approved by the Institutional Research Ethics Committee (nº 3.857.143), which waived the need for informed consent due to the retrospective study design.
We evaluated the overall prevalence of pathogens retrieved from the blood cultures of patients who had undergone systemic chemotherapy between January 2012 and January 2021 at Hospital de Clínicas de Porto Alegre, Brazil, a 919-bed, tertiary care, hybrid-founded teaching hospital. This analysis was followed by a retrospective case-control study to explore the risk factors for CRGN BSI.
Patients aged ≥18 years who had undergone chemotherapy (before or during hospitalization) and had blood cultures collected were screened for this study through a query in the hospital flowchart informatic system. Our institutional protocol recommends the collection of two blood culture samples during a febrile episode and follows international guideline recommendations for empiric antimicrobial therapy.6 For pathogen prevalence analysis, all positive blood cultures were included. For case-control analysis, we only included the hospitalized patients with FN since they were at a higher risk of complications and were most likely to benefit from a risk-guided adjustment of empirical antimicrobial therapy.7 We reviewed patient's flowchart individually for the ones included in the case-control analysis. Patients with BSI caused by CRGN bacteria were included in the study as cases. Two subjects who also underwent systemic chemotherapy and had blood cultures without isolation of CRGN bacteria (either with negative blood cultures, gram-positive bacteria, or carbapenem-susceptible gram-negative bacteria isolated) were selected as controls for each case. Controls were matched to cases by sex and year of hospital admission to balance the epidemiological prevalence of CRGN bacteria in the hospital and available healthcare resources in the same time period for cases and controls. If there was no patient to be matched in the control group in the same year, we allowed a maximum of two years difference in the date of the FN episode between cases and controls. Patients were excluded if they were not diagnosed with FN at the time of BSI.
Patients were included in the case-control analysis in the first day of FN, which was defined as the time when the absolute neutrophil count dropped to <500 cells/mm3 after systemic chemotherapy or <1000 cells/mm3 and was expected to decrease to <500 cells/µL in 48 h, with at least one episode of axillary temperature >38.3 °C or >38 °C constant for an hour.6 The first day of BSI was considered the day which blood samples that yielded positive culture results were drawn. Variables potentially related to CRGN BSI include the demographic variables (age and sex), comorbidities, Charlson comorbidity score,8 type of malignancy, chemotherapy setting (ambulatory or in-hospital), time from hospitalization to chemotherapy (days), duration of neutropenia (days), infection severity (invasive mechanical ventilation, use of vasopressor) assessed at baseline, Intensive Care Unit (ICU) admission at baseline, previous isolation of CRGN bacteria (<1 year), and hospitalization in the previous year. Active antimicrobial therapy was defined as the administration of at least one antibiotic for which the recovered bacteria showed in-vitro susceptibility.
Routine samples were identified using either the Vitek® automated system or MALDI-TOF mass spectrometry (bioMérieux, France). KPC and NDM carbapenemases were identified by genotypic testing.9 Results were interpreted according to the Clinical and Laboratory Standards Institute (CLSI) guidelines.10
Statistical analyses were performed using SPSS for Windows, Version 18.0. We calculated the median and percentiles (p)25th and 75th (p25-p75) for ordinal or non-normally distributed variables, mean and Standard Deviation (SD) for continuous variables of normal distribution, and total and percentage values for categorical variables. In addition, a univariate analysis comparing the baseline characteristics between cases and controls was performed using Fisher's exact test for categorical variables and Student's t-test or Mann-Whitney U test for continuous variables. All the tests were two-tailed, and a p-value ≤0.05 was considered statistically significant.
Cox regression model was used to assess the independent risk factors for CRGN BSI in patients with FN. Since we could not assess the outcome after hospital discharge; we chose to perform a survival analysis that allowed censoring patients at hospital discharge or 30 days, which came first. Variables with p < 0.20 in the univariate analysis were included in the stepwise-backward model, and variables with p < 0.05 were retained in the model. The variables were tested for collinearity.
For secondary outcomes, we evaluated the use of active antimicrobials for cases and controls and the impact of CRGN bacterial infections on 30-day and in-hospital mortality.
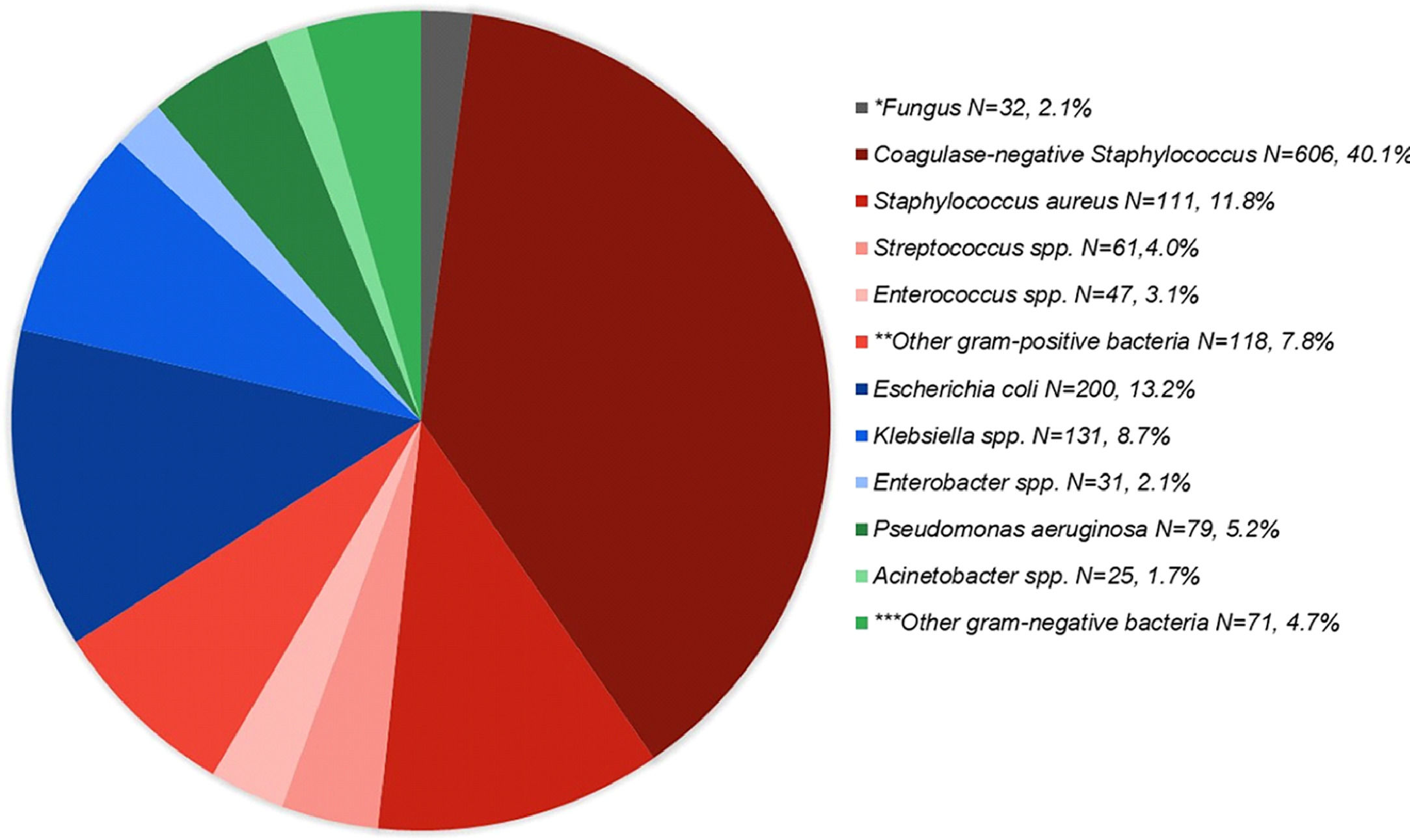
A total of 6094 blood cultures were collected from 1865 patients aged ≥ 18 years who underwent systemic chemotherapy during the study period, of which 1512 (24.8%) showed the presence of identified pathogens. Coagulase-negative Staphylococcus was the most frequently isolated pathogen (40.1%), followed by Escherichia coli (13.2%). Pathogens prevalence is shown in Fig. 1. GNB were the etiological agent in 537 (35.5%) BSI episodes. Of these, 93 (17.3%) were carbapenem-resistant: 69 of 416 (16.6%) Enterobacterales isolates and 24 of 107 (22.4%) non-fermentative bacterial isolates. Thirty-five (37.6%) patients with CRGN BSI had FN at the time of diagnosis and were included in the case-control analysis. These patients were matched to 70 controls. None of these patients had polymicrobial infections. Chemotherapy regimens are described in supplementary Table 1.
Pathogens recovered from blood cultures of patients who had undergone chemotherapy for cancer treatment (n = 1512). *25 Candida spp., 6 Cryptococcus spp., 1 Scedosporium prolificans. ** 61 Streptococcus spp., 47 Enterococcus spp., 31Corynebacterium spp., 6 Micrococcus spp., 2 Listeria monocytogenes, 1 Lactococcus lactis, 1 Rothia mucilaginosa, 1 Granulicatella adiacens, 76 unidentified. ***15 Serratia spp., 14 Proteus spp., 12 Citrobacter spp., 7 Morganella morganii, 3 Campylobacter jejuni, 3 Moraxella spp., 2 Salmonella sp., 2 Haemophilus influenzae, 2 Aeromonas spp., 1 Capnocytophaga sputigena, 1 Ochrobactrum anthropi, 1 Neisseria sp., 1 Burkholderia cepacia complex, 1 Ralstonia mannitolilytica, 1 Pasteurella multocida., 5 unidentified.
Variables significantly related to the diagnosis of CRGN BSI in univariate analysis were hematological cancer diagnosis (100% vs. 81.4%, p < 0.01), ICU admission at baseline (54.3% vs. 4.3%, p < 0.01), mechanical ventilation support at baseline (36.1% vs. 1.4%, p < 0.01), vasopressor use at baseline (28.6% vs. 10.0%, p < 0.01), chemotherapy treatment done in the hospital setting (97.1% vs. 62.9%, p < 0.01), a longer period of hospitalization before the beginning of FN (17 [12‒26] days vs. 4.5 [0‒16] days, p < 0.01), longer neutropenia duration (median of 10(5-14) days vs. 4(3-9) days, p < 0.01), and isolation of CRGN bacteria in clinical or surveillance samples in the previous year (34.3% vs. 1.4%, p < 0.01). As shown in Table 1, the other analyzed factors did not show a statistically significant difference.
Univariate analysis of patients with febrile neutropenia comparing baseline characteristics of cases (patients with isolation of CRGN bacteria from blood cultures) with controls (patients who did not isolate CRGN bacteria), matched by age and year of hospitalization and adjusted Cox regression model evaluating risk factors for the development of CRGN infections
| Univariate analysis | Multivariate analysis | ||||
|---|---|---|---|---|---|
| Cases (35) | Controls (70) | P | aHR (95%CI) | p | |
| Demographic data | |||||
| Age | 45.3 ± 13.6 | 48.2 ± 16.4 | 0.40 | ||
| Sex (masculine) | 18 (51.4) | 36 (51.4) | 0.99 | ||
| Comorbidities | |||||
| Cardiovascular disease | 12 (34.3) | 22 (31.4) | 0.83 | ||
| Diabetes | 3 (8.6) | 4 (5.7) | 0.68 | ||
| Lung disease | 3 (8.6) | 2 (2.9) | 0.33 | ||
| Central nervous system disease | 1 (2.9) | 1 (1.4) | 0.99 | ||
| HIV | 2 (5.7) | 9 (12.9) | 0.33 | ||
| Liver disease | 1 (2.9) | 4 (5.7) | 0.66 | ||
| Digestive tract | 0 | 2 (2.9) | 0.55 | ||
| Connective tissue | 0 | 3 (4.3) | 0.55 | ||
| Renal chronic disease | 2 (5.7) | 2 (2.9) | 0.60 | ||
| Charlson | 2 (2‒3) | 2.5 (2‒4) | 0.07 | ||
| Isolated Bacteriaa | |||||
| None | 0 | 61 (87.1) | |||
| CN Staphylococcus | 0 | 5 (7.1) | |||
| Streptococcus spp. | 0 | 1 (1.4) | |||
| Klebsiella pneumoniae | 24 (65.8) | 2 (2.9) | |||
| Acinetobacter baumannii | 7 (20.0) | 0 | |||
| Pseudomonas aeruginosa | 3 (8.6) | 0 | |||
| E. coli | 1 (2.9) | 1 (1.4) | |||
| Type of Malignancy | 0.28 | ||||
| Acute Myeloid Leukemia | 21 (60) | 33 (47.1) | |||
| Acute Lymphoid Leukemia | 4 (11.4) | 8 (11.4) | |||
| Chronic Lymphoid Leukemia | 1 (2.9) | 4 (5.7) | |||
| Multiple Myeloma | 1 (2.9) | 2 (2.9) | |||
| Non-Hodgkin Lymphoma | 8 (22.9) | 10 (14.3) | |||
| Breast Cancer | 0 (0) | 7 (10.0) | |||
| Colon and Rectum Cancer | 0 (0) | 5 (5.7) | |||
| Synovial Sarcoma | 0 (0) | 1 (1.4) | |||
| Hematological cancer vs. solid organ cancer | 35 (100) | 57 (81.4) | <0.01 | ||
| Chemotherapy | |||||
| First chemotherapy session | 15 (42.9) | 17 (24.3) | 0.07 | 3.3 (1.37‒8.02) | <0.01 |
| Chemotherapy setting (hospital) | 34 (97.1) | 44 (62.9) | <0.01 | 8.9 (1.21‒65.50) | 0.03 |
| Time from hospital admission to FN (days) | 17 (12‒26) | 4.5 (0‒16) | <0.01 | ||
| FN duration (days) | 10 (5‒14) | 4 (3‒9) | <0.01 | ||
| Hospitalization | |||||
| Year of hospitalization | 2016 (2015‒2018) | 2016 (2015‒2019) | 0.94 | ||
| Mechanical ventilation | 13 (36.1) | 1 (1.4) | <0.01 | ||
| Vasopressor | 10 (28.6) | 0(0) | <0.01 | ||
| Intensive care unit admission | 19 (54.3) | 3(4.3) | <0.01 | 2.8 (1.34‒5.71) | <0.01 |
| Previous isolation carbapenem-resistant bacteria (<1 year) | 12 (34.3) | 1 (1.4) | <0.01 | ||
| Previous hospitalization (<1 year) | 22 (62.9) | 60 (85.7) | 0.95 | 5.5 (2.06‒14.70) | <0.01 |
aHR, adjusted Hazard Ratio; CI, Confidence Interval; CN, Coagulase-Negative; CRGN, Carbapenem resistant gram-negative; FN, Febrile Neutropenia.
In multivariate analysis, significant risk factors for the development of CRGN BSI within 30-days following the beginning of FN were: first chemotherapy session (p < 0.01); chemotherapy performed in the hospital setting (p = 0.03); ICU admission at baseline (p < 0.01), and CRGN bacteria isolated in the previous year (p < 0.01), as shown in Table 1.
In the case group, 15 (42.9%) patients received cefepime as the first antimicrobial therapy after FN, 18 (51.4%) were administered meropenem, 1 (2.9%) patient received amikacin plus colistin, and 1 (2.9%) received ceftazidime. As the second most used antibiotic, 19 (54.3%) patients received vancomycin. Only one patient in the case group received active antimicrobial therapy for CRGN bacteria as the first choice after the diagnosis of FN. Active antibiotics were eventually prescribed to 22 (62.9%) patients in the case group at a median time of 1 (0‒3) day after the first day of BSI. The remaining 13 (37.1%) patients did not receive any active antibiotics. The antibiotics used for carbapenem-resistant infections were: polymyxins, 11 (31.4%); aminoglycosides, 10 (28.6%); and tigecycline, 1 (2.9%).
In the control group, 64 (91.4%) patients were administered cefepime, 4 (5.7%) were given meropenem, 1 (1.4%) ceftazidime, and 1 (1.4%) was started on vancomycin. Seven (77.8%) of the nine patients in whom bacteria were isolated in blood cultures from the control group received active antimicrobials as the first choice after FN. The two patients in this group who did not receive active antimicrobials had isolated coagulase-negative Staphylococcus.
Mortality within 30 days occurred in 10 of 35 (28.6%) cases vs. 1 of 70 (1.4%) controls (p < 0.01). In-hospital mortality occurred in 22 of 35 (62.9%) cases vs. 2 of 70 (2.9%) controls (p < 0.01).
Gram-positive bacteria were the most frequently identified pathogens in our study. This could have been influenced by use of central-venous catheters by many of these patients. We did not assess the clinical significance of coagulase-negative Staphylococcus BSI, and it could represent blood sample contamination in some of the cases.11 GNB was the second most prevalent etiology (35.5%). Our data are in accordance with a previous systematic review which found bacterial infections to be the most common complication in cancer patients with GNB isolation accounting for 24.7%‒73.9% of the cases.12 In a recent multinational European Surveillance Program that evaluated pathogens causing BSI in 9080 patients with hematologic cancer, a 16% proportion of positive blood cultures were found,13 which was lower than in our sample (24.8%). In that study the most isolated bacteria were also coagulase-positive Staphylococcus (38%), followed by E. coli (19%).13
Latin America has high rates of CRGN bacterial infection cases14; however, data regarding patients undergoing cancer treatment is still relatively scarce.7 In this study, we found a significant proportion of GNB BSI among our patients, with particularly high rates of carbapenem resistance among Enterobacterales (16.6%). In a study with 591 hematopoietic stem cell transplant recipients from 25 European countries, carbapenem resistance accounted for 8.4% of Enterobacterales BSI.12
All patients who developed CRGN bacterial BSI during FN episodes had hematological malignancies. High chemotherapeutic doses, longer hospitalization time, and prolonged neutropenia periods in hematological cancer patients may explain the higher proportion of infections in this group. A previous study including 637 patients with oncological diseases found a 98.1% prevalence of hematological malignancies in patients presenting with bloodstream infections.12 In our study, after matching patients with CRGN bacterial infections to controls, the first chemotherapy session was found to be an independent risk factor for CRGN bloodstream infections. Our hypothesis to explain this finding is that patients have a more severe presentation of the oncological disease during the first chemotherapy session (or induction chemotherapy) than patients in the following sessions, which could have contributed to their higher vulnerability to CRGN bacterial infections. We also found that chemotherapy treatment performed in the hospital setting and ICU admission at the beginning of FN were both associated to higher rates of CRGN BSI, which translates exposure to settings with a higher prevalence of these bacteria.15 Finally, the isolation of CRGN bacteria in the previous year may indicate that the patient could still be colonized, thus, increasing the risk of infection during chemotherapy-induced immunosuppression.15
Thirty-day and in-hospital mortality rates were significantly higher in the case patients than in the controls. Weber et al. classified BSI in cancer patients according to the 30-day mortality outcome. In their study, patients with multidrug-resistant GNB with additional carbapenem resistance showed the highest mortality risk.12 In an 11-year cohort of 552 patients with FN and BSI, the mortality rate of patients who isolated CRGN bacteria was 65.5%, significantly higher than in those patients with other etiological agents.4
The low proportion of patients receiving active antimicrobials and the delay in appropriate therapy in the case group, as shown in our study, may explain the higher mortality rate observed in these cases.7 In accordance with the current international guideline recommendations,6 empirical antimicrobial therapy prescribed after FN diagnosis in our study was mostly based on cefepime ‒ or meropenem-containing regimens, with the addition of vancomycin in a small proportion of cases. Only one of the patients with CRGN BSI received empirical active antimicrobial therapy, and 62.9% eventually received it during hospitalization. A lower proportion of patients receiving active antimicrobials when infected with CRGN bacteria were also found in a large cohort of bone marrow transplant patients, wherein 63.9% received inappropriate empirical antimicrobial therapy in the group with CRGN bacterial infections as compared to 12.7% in patients with carbapenem-susceptible infections.16 Rosa et al. evaluated FN patients and reported that every 1 h delay in antibiotic prescription increased mortality risk by 18% within 28 days.7 This highlights the need to identify high-risk patients for carbapenem-resistant infections and the prompt initiation of antimicrobials with activity against these bacteria. [17]
This study had some limitations, such as its retrospective nature and the inclusion of single-center data. The relatively small number of CRGN BSI and the low mortality rate in the control group limited the power of multivariate analysis for some of the included variables. Moreover, most of the patients in our sample had acute myeloid leukemia, which can somewhat compromise external validity, since these patients commonly present with more severe disease and receive more aggressive chemotherapy regimens, compared to other types of cancer. Despite these limitations, this study translates a 10-years epidemiology analysis, which is the largest study conducted in Latin America to evaluate BSI pathogen prevalence in patients undergoing systemic chemotherapy for cancer treatment. It appoints risk factors for CRGN bacterial infections, which should be considered for individualizing the choice of empirical antibiotic therapy in FN patients.