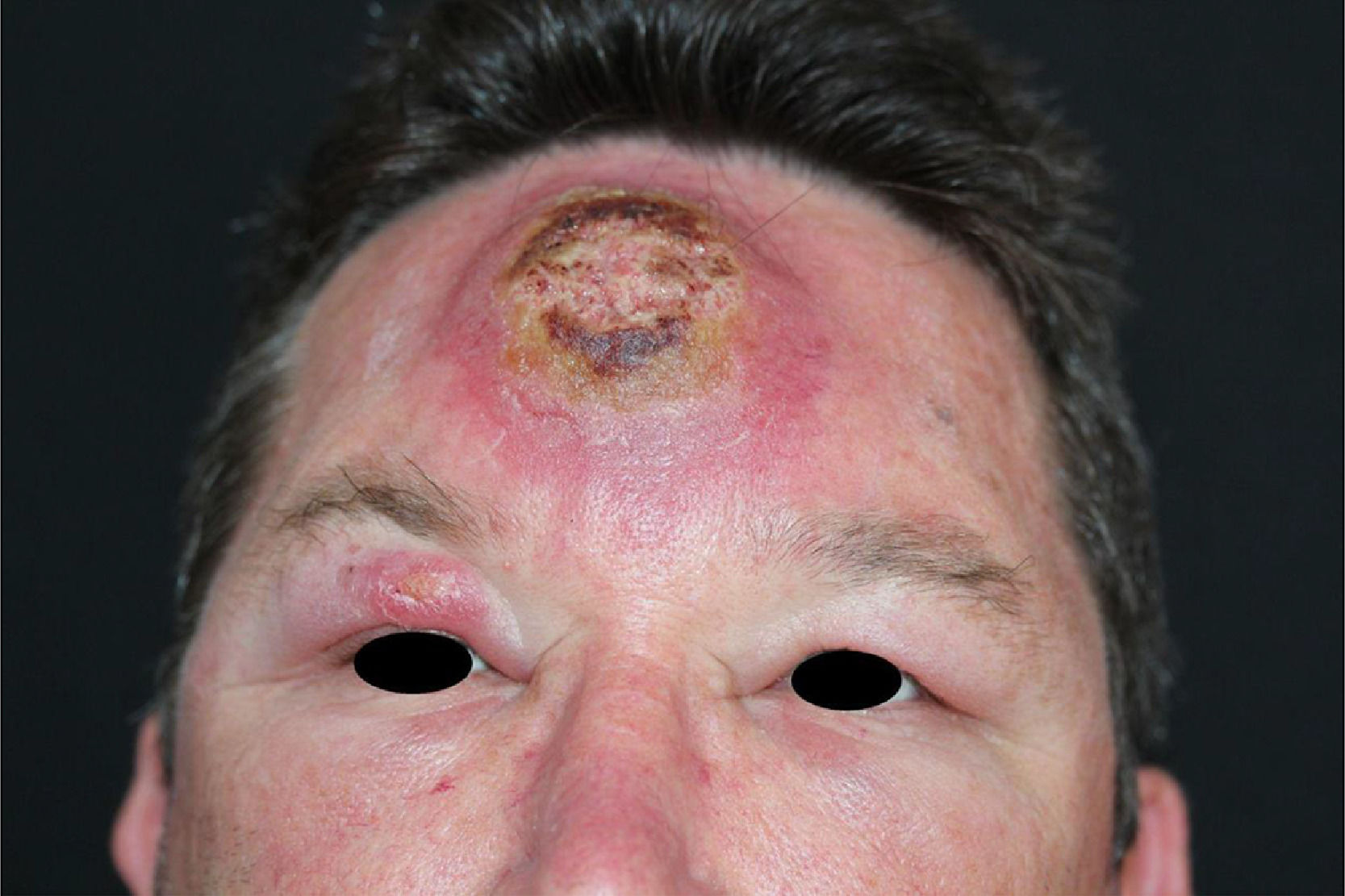
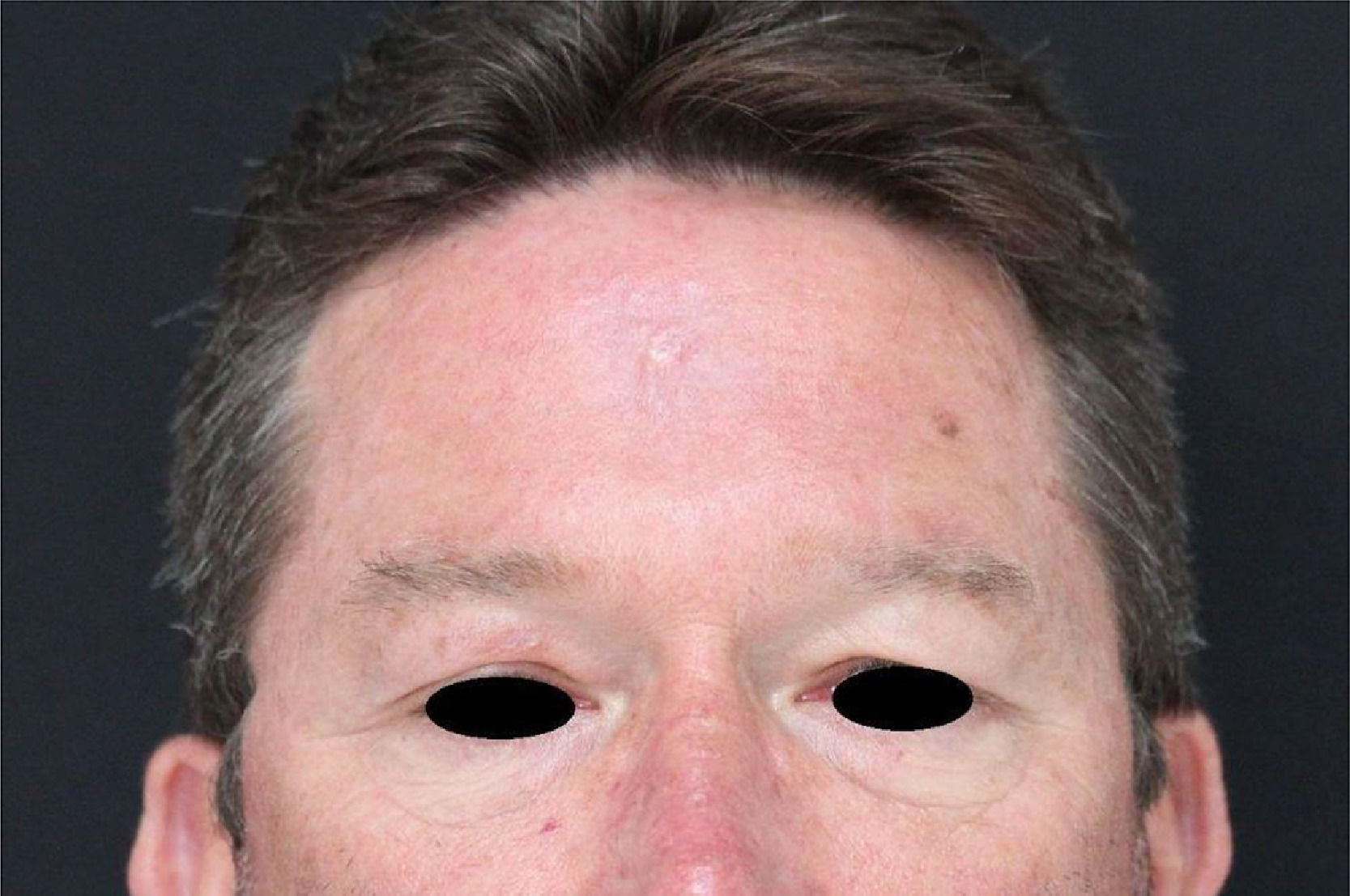
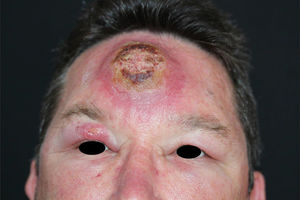
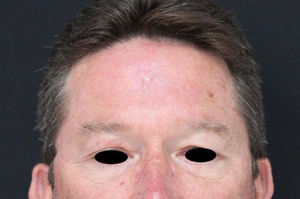
Leishmaniasis is a global disease, with most of the cases seen in tropical regions: South America, Asia, Africa, and the Mediterranean.1 It is caused by a protozoa of the genus Leishmania, which is transmitted to humans by the bite of female phlebotomine sandflies.2 There are three main clinical forms of leishmaniasis: visceral, mucocutaneous and cutaneous - the latter presentation begins with a small papule at the inoculation site, usually at an exposed area of the body.3 Herein we report a case of a previously healthy patient, resident in a rural area, who works as a truck driver transporting tree trunks. Three months prior to the dermatological consultation the patient noted an inflammatory lesion on the forehead, with progressive growth and an adjacent erythematous halo, later with the appearance of a lesion on the upper right eyelid. The patient had undergone surgical drainage and received non-steroidal anti-inflammatory drugs and antibiotics without improvement, prescribed by his general practitioner. On examination, an ulcerated nodule measuring approximately 8 × 6 cm is noted, associated with swelling of the forehead and bilateral eyelid region. There was also an ulcerated nodule in the upper right eyelid, with an indurated tract extending posteriorly towards the ipsilateral temple. No signs of mucosal involvement on eyelid eversion. As diagnostic hypotheses, the PLECT group of diseases was considered - paracoccidioidomycosis, leishmaniasis, sporotrichosis, chromoblastomycosis and cutaneous tuberculosis. Direct microscope examination (imprint) showed many amastigote forms, and histopathology was compatible with American Cutaneous Leishmaniasis (Fig. 1). He was admitted to the Dermatology Inpatient Clinic and received 20 days of glucantime 4.5 g/daily under heart and vital sign monitoring, associated with pentoxifylline 400 mg 8/8 hours, progressing to clinical cure. The patient remains with no signs of recurrence after 18 months of follow-up, with only a small scar on the forehead (Fig. 2).
Data confidentialityThe authors declare having followed the protocols in use at their working center regarding patients’ data publication.
Informed consentObtained.
No funding. Authors were responsible for all the costs involved in this article.