The prevalence of sarcopenia in hospitalized people living with HIV is underdiagnosed, as assessment instruments are not always available. This study aimed to identify factors related to sarcopenia, correlating their anthropometric and clinical markers in hospitalized people living with HIV. This was an observational cross-sectional clinical study, carried out from September 2018 through October 2019. Handgrip strength, muscle mass index, calf circumference and gait speed test were evaluated in recruited patients within three days of hospital admission. The sample consisted in 44 patients, mostly men (66%), black (68%), young adults (41.65±12.18 years) and immunodeficient (CD4 cell count 165 cells/mm3 [34.25–295.5]). Sarcopenia was present in 25% of the sample. Calf circumference showed a significant correlation with CD4 cell count and viral load (p<0.05) while handgrip strength and gait speed test did not. Calf circumference>31cm and gait speed test>0.8m/s reduced the chance of sarcopenia by 60% (OR=0.396 [−1.67 to −0.18]; p<0.05) and 98% (OR=0.02 [−8.16 to 0.13]; p<0.05) respectively. Calf circumference>31cm and gait speed test>0.8m/s are associated with a reduced chance of sarcopenia in hospitalized HIV patients.
Sarcopenia is a progressive and generalized muscle disorder related to aging and chronic diseases.1 People living with human immunodeficiency virus (PLHIV) still have a great loss of muscle mass and strength. Nonetheless, HIV is not recognized as a risk factor for sarcopenia. The prevalence of sarcopenia in PLHIV is 24% and there is a six-fold greater chance of sarcopenia in this population when compared to people without the virus.2
Sarcopenia in PLHIV seems to occur earlier, around 15 years before in the population without HIV.2 The immune dysfunction caused by constant viral replication, the persistent inflammatory state, and the toxicity of antiretroviral therapy (ART) corroborate the theory of “accelerated aging syndrome” in PLHIV,3,4 that is, premature appearance of changes in the cardiovascular, renal, cognitive, and bone systems.
Hospitalization of PLHIV is still frequent either for immunodeficiency or for diseases unrelated to aids (acquired immunodeficiency syndrome), which may favor the development of sarcopenia or worsen the pre-existing condition. Acute sarcopenia is the loss of muscle mass and strength associated with hospitalization,5 and is associated with longer hospital stays, hospital readmissions, increased health costs and greater need for post-discharge assistance.6,7 However, the frequency of sarcopenia in this environment is scarce in the literature.5
The aims of this study were to identify factors related to sarcopenia in hospitalized HIV-infected patients and correlate with their anthropometric and clinical markers.
This was an observational and cross-sectional clinical study, carried out in the city of Rio de Janeiro, Brazil, from September 2018 through October 2019. All patients signed the Free Consent Form and Clarified. The project was approved by the Institution's Research and Ethics Committee with reference CAAE: 89594318.6.0000.5258.
The sample consisted of patients with HIV infection, aged 18 years or older, ability to understand and execute simple commands, hemodynamic stability, orthostatic capacity and evaluation within 72h of hospitalization. Patients with pain, dyspnea or any cardiopulmonary alteration that could interfere with the physical tests, progressive neuromuscular diseases, pregnant women, obese patients (BMI>30kg/m2), disabling osteomioarticular and skin lesions, and bedridden patients were excluded.
Assessment and staging of sarcopenia were performed as suggested by the European Working Group on Sarcopenia in Older People.1 Patients were classified into two groups: the non-sarcopenic (NS) group comprised patients with no change in muscle strength and those with reduced muscle strength only (probable sarcopenia); and the Sarcopenic group (S), patients who presented a reduction in muscle strength and muscle mass (sarcopenia) and a reduction in muscle strength, muscle mass and physical performance (severe sarcopenia).
Muscle strength was measured using the Manual Grip Force (HGS), with the hydraulic hand dynamometer (SH Hydraulic Hand Dynamometer - Saehan®; Massan, Korea). The largest measure of three assessments in the dominant limb was used. The criteria for defining reduced muscle strength are HGS values<16kg in female and<27kg in male.1
Muscle mass was measured using octapolar electrical bioimpedance (Bioimpedance scale HBF 514C - Omron®; Kyoto, Japan). The scale provided the percentage value of skeletal muscle mass (SMM), which was converted into an absolute number after multiplying the observed value by the individual's total weight, also provided by the scale. The SMM was divided by the square of body height to obtain the Muscle Mass Index (MMI). The indicative criteria for reducing skeletal muscle mass are MMI values≤5.5kg/m² for women and≤7kg/m² for men.1
The calf circumference (CC) of the right lower limb was measured with the patient seated and feet flat on the floor, using a tape measure; a value<31cm is indicative of reduced SMM.1
Physical performance was analyzed using the 4-m gait speed test (GST), in which the patient traveled twice, the distance at the highest possible speed, and the highest value obtained was used. The value≤0.8m/s is considered as poor physical performance.1
Statistical analysis was performed using the statistical package JASP 0.11.1 (Amsterdam, 2019). Continuous data were tested using the Shapiro–Wilk normality test to determine their distribution. The normally distributed data were expressed as mean±standard deviation (SD), and non-normal data through medians and interquartile ranges (IQR) or minimum–maximum range. The t test and Mann–Whitney rank sum test were used to compare two means of continuous parametric and nonparametric variables, respectively. Pearson’s or Spearman’s coefficients were used for the possible linear correlations of parametric and nonparametric continuous variables, respectively. Anthropometric and clinical measures were analyzed using binary logistic regression in order to identify variables associated with sarcopenia, assuming p<0.1 as the model variable. A p value<0.05 was considered for statistical significance.
For sample size calculation, we considered the number of HIV patients admitted to the wards during the study period, totaling 60 patients and frequency of sarcopenia in stable PLHIV of 24% (Oliveira et al., 2020). Considering a 95% confidence interval and 5% absolute precision, the required sample size was 50 patients (OpenEpi, 2013).
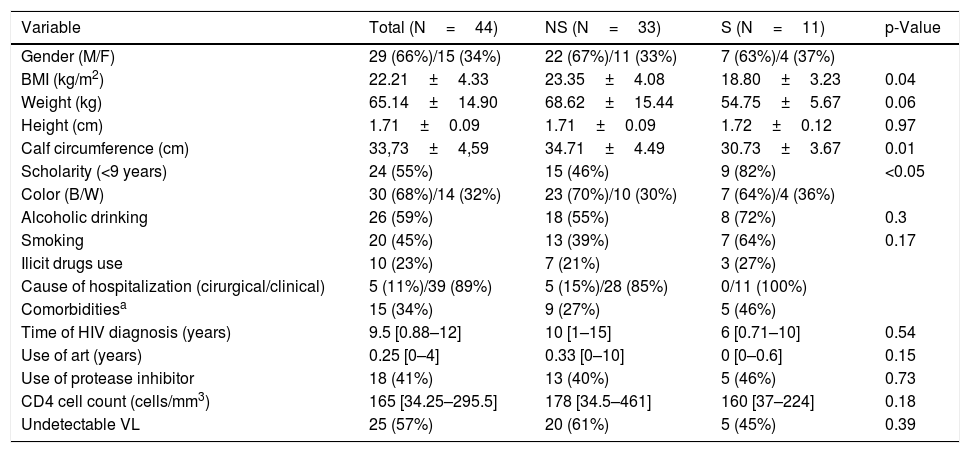
Were eligible for the study 44 patients, 11 (25%) were sarcopenic. The demographic, clinical and anthropometrical data of the sample are shown in Table 1. The sample consisted predominantly of young adults (41.65±12.18 years), males (66%) and blacks (68%). The reasons for hospitalization in 89% of the sample were due to clinical causes, and of these, 59% due to opportunistic infections. The median CD4 cell count was 165 [34.25–295.5] cells/mm3 and 64% of patients used ART for less than one year or used it irregularly.
Demographic, clinical and anthropometrical characteristics of the patients.
| Variable | Total (N=44) | NS (N=33) | S (N=11) | p-Value |
|---|---|---|---|---|
| Gender (M/F) | 29 (66%)/15 (34%) | 22 (67%)/11 (33%) | 7 (63%)/4 (37%) | |
| BMI (kg/m2) | 22.21±4.33 | 23.35±4.08 | 18.80±3.23 | 0.04 |
| Weight (kg) | 65.14±14.90 | 68.62±15.44 | 54.75±5.67 | 0.06 |
| Height (cm) | 1.71±0.09 | 1.71±0.09 | 1.72±0.12 | 0.97 |
| Calf circumference (cm) | 33,73±4,59 | 34.71±4.49 | 30.73±3.67 | 0.01 |
| Scholarity (<9 years) | 24 (55%) | 15 (46%) | 9 (82%) | <0.05 |
| Color (B/W) | 30 (68%)/14 (32%) | 23 (70%)/10 (30%) | 7 (64%)/4 (36%) | |
| Alcoholic drinking | 26 (59%) | 18 (55%) | 8 (72%) | 0.3 |
| Smoking | 20 (45%) | 13 (39%) | 7 (64%) | 0.17 |
| Ilicit drugs use | 10 (23%) | 7 (21%) | 3 (27%) | |
| Cause of hospitalization (cirurgical/clinical) | 5 (11%)/39 (89%) | 5 (15%)/28 (85%) | 0/11 (100%) | |
| Comorbiditiesa | 15 (34%) | 9 (27%) | 5 (46%) | |
| Time of HIV diagnosis (years) | 9.5 [0.88–12] | 10 [1–15] | 6 [0.71–10] | 0.54 |
| Use of art (years) | 0.25 [0–4] | 0.33 [0–10] | 0 [0–0.6] | 0.15 |
| Use of protease inhibitor | 18 (41%) | 13 (40%) | 5 (46%) | 0.73 |
| CD4 cell count (cells/mm3) | 165 [34.25–295.5] | 178 [34.5–461] | 160 [37–224] | 0.18 |
| Undetectable VL | 25 (57%) | 20 (61%) | 5 (45%) | 0.39 |
NS: non- sarcopenic; S: sarcopenic; M: male; F: female; B: black; W: white; ART: antiretroviral therapy; VL: viral load.
Of the total of the sample, 59% had reduced muscle strength, 39% reduced muscle mass and 41% reduced physical performance. In comparison to the non-sarcopenic group, the sarcopenic group showed significantly lower values for BMI (NS 23.35±4.08 X S 18.80±3.23kg/m2; p<0.01), MMI (NS 8.11±1.56 X S 5.34±1.09kg/m2; p<0.01), CC (NS 34.71±4.49 X S 30.73±3.67cm; p<0.01), HGS (NS 23.82±8.52 X S 16.64±4.72; p<0.01) and GST (NS 1.01±0.33 X S 0.75±0.22m/s; p<0.05).
Based on sarcopenia stages, 41% did not have sarcopenia, 34% had probable sarcopenia, 7% had sarcopenia and 18% severe sarcopenia. There was no significant difference between the staging performed by bioimpedance and CC (41%, 32%, 5%, 22% respectively) (p>0.05).
There was a significant correlation between the different muscle mass tests (r=0.47; p=0.002). Furthermore, there was a correlation between muscle mass and muscle strength (Bioimpedance r=0.51; p=0.002; CC r=0.43; p=0.004), muscle mass and physical performance (Bioimpedance r=0.39; p=0. 03; CC r=0.37; p=0.02) and muscle strength and physical performance (r=0.57; p<0.001).
CC showed a significant correlation with viral load (r=−0.32; p<0.05) and CD4 cell count (r=0.34; p<0.05); such correlation was not observed for Bioimpedance, HGS and GST. The use of protease inhibitors was also not correlated with anthropometric variables. Presence of sarcopenia had a significant correlation with the CD4 cell count (r=−0.369; p<0.05).
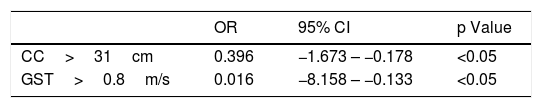
In the logistic regression analysis, calf circumference greater than 31cm reduced the chance of sarcopenia by approximately 60% (OR=0.396 [−1.673 to −0.178], while gait speed greater than 0.8m/s reduced the chance in 98% (OR=0.016 [−8.158 to −0.133] (Table 2).
The present study identified a frequency of 25% of sarcopenia in a sample of individuals with HIV/aids admitted to an HIV referral hospital in Rio de Janeiro. In addition, 34% of the sample had reduced muscle strength, which alarms the high possibility of developing sarcopenia in this population.
In the present study, the median time of HIV infection was 9.5 years, but 64% of patients used irregularly or recently started antiretrovirals, which indicates low adherence to treatment. This explains the finding of a significant percentage (75%) of patients with a CD4 cell count below 350 cells/mm³ and a significant number of hospitalizations for opportunistic infections (59%), which characterizes the presence of aids.
Our findings are consistent with those described in the literature for PLHIV. Oliveira et al. in a recent meta-analysis on the topic, included 13 studies, totaling 2267 patients, mostly men (76%), aged between 35 and 60 years, on ART, with undetectable VL and CD4 cell count>500 cells/mm³. The prevalence of sarcopenia among PLHIV was 24.1% (95% CI=17.8–31%). Still, this group had 6.1 greater odds of sarcopenia (95% CI=1.1–33.5) compared to people without HIV, matched by age, sex, BMI and ethnicity.2
There is no description in the literature of reference values for muscle mass and muscle strength for PLHIV. Thus, the reference values used were those suggested by the European Working Group on Sarcopenia in Older People1 and, even so, the results of our relatively young sample were lower. These findings underscore the hypothesis that sarcopenia in PLHIV develops earlier due to aging of the immune system, caused by chronic systemic inflammatory process, virus infection, and antiretroviral therapy.8
According to Yarasheski et al., there is a correlation between CD4 cell count and muscle mass measured by dual-energy X-ray absorptiometry (DEXA),9 which was not observed in the study by Echeverria.10 Abdul Aziz reports a 1% reduction in the risk of sarcopenia with each unit increase in CD4 cell count in the Asian population.11 It is also described that a longer infection time can predispose to muscle mass loss.10 In our study, sarcopenic patients had lower CD4 cell count and higher viral load compared to non-sarcopenics, however this value was not significant, which can be justified by the small sample size.
When analyzing anthropometric variables, only muscle mass assessed through the measurement of calf circumference was correlated with viral load and CD4 cell count. We also observed that calf circumference measurement greater than 31cm and gait speed greater than 0.8m/s were protective factors for sarcopenia in hospitalized PLHIV. These data are important because they demonstrate that simple tests can be used to assess and monitor this population during hospitalization.
Calf circumference measure has been used to estimate muscle mass in the elderly in clinical practice and has a good correlation with gold standard methods even for younger populations.12,13 Studies indicate that the measure is able to predict hospital readmission in adults14 and survival among the elderly.15 Our study found correlation between calf circumference and the variables related to HIV, in addition to similar results in the staging of sarcopenia when compared to bioimpedance.
Reduced gait speed is associated with functional decline, disability and death.16 PLHIV are at risk of 30% reduction in physical performance, and the association between the presence of the virus and reduced physical performance increases mortality by six times.17 Schrack et al. used the 4-m gait speed test in PLHIV aged 40 years and over and concluded that there was faster and earlier gait decline in this population, with a 57% risk of developing reduction in walking speed,18 also observed in our study,
The present study had some limitations. The cross-sectional and observational nature of the study and the non-inclusion of a control group prevented demonstration of a causal relationship and a direct comparison between frequencies of hospital sarcopenia in the HIV negative population. As we were not able to enroll the required sample size, we were left with a convenience sample. The limited number of participants also hampered further comparisons between groups. In addition, lack of financial resources prevented the use of high precision evaluation instruments.
In conclusion, in the population with HIV/aids admitted to a referral hospital for HIV, consisting mostly of men, black, young adults and immunodeficient people with low adherence to treatment, the frequency of sarcopenia was 25%. Sarcopenia was correlated with CD4 cell count in this population. The calf circumference was significantly correlated with electrical bioimpedance and may be used to measure muscle mass in these patients, in addition to significantly correlating with viral load and CD4 cell count. Furthermore, calf circumference>31cm and walking speed>0.8m/s suggest a reduction in the chance of sarcopenia in this population.
Conflicts of interestThe authors declare no conflicts of interest.
FundingNothing to declare.