Infection with Gram-negative bacteria is associated with increased morbidity and mortality. The aim of this study was to evaluate the predictors of 7- and 30-day mortality in pediatric patients in an intensive care unit with cancer and/or hematologic diseases and Gram-negative bacteria infection.
MethodsData were collected relating to all episodes of Gram-negative bacteria infection that occurred in a pediatric intensive care unit between January 2009 and December 2012, and these cases were divided into two groups: those who were deceased seven and 30 days after the date of a positive culture and those who survived the same time frames. Variables of interest included age, gender, presence of solid tumor or hematologic disease, cancer status, central venous catheter use, previous Pseudomonas aeruginosa infection, infection by multidrug resistant-Gram-negative bacteria, colonization by multidrug resistant-Gram-negative bacteria, neutropenia in the preceding seven days, neutropenia duration ≥3 days, healthcare-associated infection, length of stay before intensive care unit admission, length of intensive care unit stay >3 days, appropriate empirical antimicrobial treatment, definitive inadequate antimicrobial treatment, time to initiate adequate antibiotic therapy, appropriate antibiotic duration ≤3 days, and shock. In addition, use of antimicrobial agents, corticosteroids, chemotherapy, or radiation therapy in the previous 30 days was noted.
ResultsMultivariate logistic regression analysis resulted in significant relationship between shock and both 7-day mortality (odds ratio 12.397; 95% confidence interval 1.291–119.016; p=0.029) and 30-day mortality (odds ratio 6.174; 95% confidence interval 1.760–21.664; p=0.004), between antibiotic duration ≤3 days and 7-day mortality (odds ratio 21.328; 95% confidence interval 2.834-160.536; p=0.003), and between colonization by multidrug resistant-Gram-negative bacteria and 30-day mortality (odds ratio 12.002; 95% confidence interval 1.578–91.286; p=0.016).
ConclusionsShock was a predictor of 7- and 30-day mortality, and colonization by multidrug resistant-Gram-negative bacteria was an important risk factor for 30-day mortality.
Children undergoing treatment for malignancy have an excellent chance of survival, with an overall survival rate approaching 75%.1 In most cases, children who die following treatment for cancer do so as a result of their disease. However, despite significant improvements in supportive care, approximately 16% of the deaths within five years following diagnosis are due to complications of therapy.1 Infection continues to be one such life-threatening complication in immunocompromised children.1
With recent advances in cancer treatments and improvements in critical care, an increasing number of patients with hematologic malignancies are being admitted to intensive care units (ICU).2 Despite the associated improvements in outcomes, mortality remains high in critically ill patients with hematologic malignancies, particularly in the presence of ICU-acquired nosocomial infections.2 In addition, infection results in ICU admission in cancer patients.
Oncologic patients are at a greater risk of developing sepsis and nosocomial infections as a consequence of a number of mechanisms, including immunosuppression related to the disease itself and to aggressive treatments, such as combined regimens of chemotherapy and radiation therapy, high dose steroids, and hematopoietic stem cell transplantation.3 Moreover, despite recent improvements in survival rates, sepsis in patients with cancer remains associated with high morbidity, mortality, costs, and use of ICU resources, and information on this topic is limited.3
Research has previously focused on adult patients with cancer. Little is known regarding the predictors of 7- and 30-day mortality related to Gram-negative bacterial (GNB) infection in children with cancer who are hospitalized in the ICU. Therefore, this study aimed at evaluating the predictors of 7- and 30-day mortality risk in children with cancer and/or hematologic diseases who were also infected with GNB.
Patients and methodsStudy designWe performed a case–control study in the pediatric ICU of the National Cancer Institute (INCA), Rio de Janeiro, Brazil, which is an exemplary tertiary oncology public hospital. The 6-bed pediatric ICU admits only patients with solid tumors and hematologic malignancies. Admission to the ICU is not generally permitted for palliative care. However, some patients may be admitted while a full assessment of the extent of their cancer and therapeutic options is still ongoing.
We collected data related to all GNB episodes that occurred between January 2009 and December 2012 in patients aged less than 18 years, who were hospitalized for >24h in the pediatric ICU. The study was approved by the ethics committee of the Fluminense Federal University.
GNB infections were divided into two groups by time and survival status: patients who were deceased and those who survived seven and 30 days after the date of a positive culture. At each time period, the patients were from the same unit and had infections that occurred at the same time and at the same sites.
The following data were collected: age, gender, presence of solid tumor or hematologic disease, cancer status, central venous catheter use, previous Pseudomonas aeruginosa infection, healthcare-associated infection, infection by multi-drug resistant (MDR)-GNB, colonization by MDR-GNB (assessed using a rectal swab or stool culture), neutropenia in the preceding seven days, neutropenia duration ≥3 days, length of stay before ICU admission, length of ICU stay >3 days, appropriate empirical antimicrobial treatment, definitive inadequate antimicrobial treatment, appropriate antibiotic duration ≤3 days, the time to initiate adequate antibiotic therapy, and shock (within two days before or after the date of a positive culture). In addition, the use of any of the following in the previous 30 days was noted: antimicrobial agents, corticosteroids, chemotherapy, or radiation therapy. Colonization with antibiotic-resistant GNB was detected via weekly routine surveillance (nasal and rectal swabs).
Definition of termsDeath within seven days or 30 days after the date of a positive culture was considered as 7- and 30-day mortality, respectively. An episode of infection was defined as the isolation of GNB from three days before the date of pediatric ICU admission to the last day of hospitalization in the pediatric ICU, from cultures of blood, urine, stools, broncho-alveolar lavage, tracheal aspirate, cerebrospinal fluid, or catheter tip, in the presence of associated, compatible clinical signs or symptoms. The tracheal aspirate was collected from endotracheal tubes and tracheostomies of patients who underwent mechanical ventilation. The diagnostic criteria for pneumonia included purulent tracheobronchial secretion and new pathogenic bacteria isolated from tracheal aspirate or broncho-alveolar lavage in addition to two or more of the following criteria: fever >38°C; leukocytes >12,000cells/mL or <4000cells/mL; a new and persistent (>48h) infiltrate on chest radiograph; new onset or worsening cough, dyspnea, or tachypnea; and declining gas exchange. Catheter cultures were performed when a catheter was removed for suspected intravascular catheter-related infection, if the patient had unexplained sepsis or erythema overlying the catheter insertion site, or if there was purulence at the catheter insertion site. The end of the infection episode was the date when the infection was considered to be resolved by the medical team.
The presence of extended spectrum beta-lactamase (ESBL)-producing Enterobacteriaceae; microorganisms with intrinsic resistance mechanisms such as S. maltophilia and Elizabethkingia meningoseptica; carbapenem-resistant GNB; or strains of A. baumannii were considered as MDR-GNB infection. Polymicrobial infection involved the isolation of more than one pathogen from a culture sample.
Neutropenia was defined as an absolute neutrophil count <500neutrophils/mm3 in blood samples. Shock was defined as arterial hypotension requiring vasoactive drugs.
Initial antimicrobial treatment was considered inappropriate if the treatment regimen did not include at least one antibiotic active in vitro against the microorganism. It was also considered inappropriate when the antibiotic treatment was not started on the date of the positive culture. Furthermore, in patients from whom ESBL-producing bacteria had grown in cultures, treatment with penicillin and cephalosporins was considered inappropriate. Definitive inadequate antimicrobial treatment was defined when the patient did not receive any antibiotic active in vitro against the microorganism during the period of infection.
Microbiological studiesBacterial identification was performed by the INCA microbiology laboratory using the Vitek automated method (Bio-Merieux, Inc., France) with manual confirmation of bacterial isolates and antimicrobial resistance, as per the standards recommended by the Clinical Laboratory Standard Institute (CLSI).
Statistical analysisStatistical analysis was conducted using SPSS v17 (SPSS Inc; Chicago, Illinois, USA). Continuous variables were compared using Mann–Whitney U test and Student's t-test. Categorical variables were analyzed using two-tailed Chi-square test. Odds ratios (OR) and 95% confidence intervals (CI) were calculated. Separate multivariate logistic regression analyses included the factors potentially associated with 7- or 30-day mortality based on statistically significant variables in univariate analyses. A value of p<0.05 was considered statistically significant.
ResultsIn the period from January 1, 2009 to December 31, 2012, there were 101 episodes of GNB infection in 76 patients out of 765 total admissions to the pediatric ICU. Of these episodes, 47 (46.5%) were related to MDR-GNB infection. Eight (10.5%) patients presented with >1 episode of non-MDR-GNB infection, 4 (5.3%) patients presented with >1 episode of MDR-GNB infection, and 8 (10.5%) patients presented with separate episodes of MDR-GNB and non-MDR-GNB infections. A single GNB was isolated in 98 (97.0%) episodes, and in three (3.0%) episodes, two isolates were recovered. All-cause mortality rate was 7.9% (8/101) on day 7 and 20.8% (21/101) on day 30.
During the study period, 18 episodes did not have a related rectal swab or stool culture (two deceased and 16 survivors); however, the characteristics, except for age, were similar between these patients and those with a swab or culture (Table 1).
Characteristics of pediatric patients with Gram-negative bacterial infection based on rectal swab or stool culture.
| With swab or culturen=83n (%) | Without swab or culturen=18n (%) | p value | |
|---|---|---|---|
| Gender (boys) | 42 (50.6) | 10 (55.6) | 0.792 |
| Age (years)a | 5 (0.2–18) | 12 (1–17) | 0.023 |
| MDR-GNB | 42 (50.6) | 6 (33.3) | 0.298 |
| Cancer status (controlled) | 15 (18.1) | 5 (27.8) | 0.262 |
| Hematologic diseases | 21 (25.3) | 3 (16.7) | 0.551 |
| Previous antibiotic therapyb | 75 (90.4) | 14 (77.8) | 0.219 |
| Central venous catheter | 76 (91.6) | 17 (94.4) | 1.000 |
| Use of corticosteroidsb | 75 (90.4) | 17 (94.4) | 1.000 |
| Neutropeniac | 23 (27.7) | 4 (22.2) | 0.774 |
| Neutropenia duration ≥3 days | 14 (16.9) | 4 (22.2) | 0.590 |
| Healthcare-associated infection (from ward and pediatric ICU) | 68 (82.0) | 14 (77.8) | 0.683 |
| Length of stay before ICU admissiona | 2 (0–89) | 2.5 (0–14) | 0.844 |
| Length of ICU stay >3 days | 42 (50.6) | 8 (44.4) | 0.636 |
| Chemotherapy/radiation therapyb | 61 (73.5) | 11 (61.1) | 0.389 |
| Previous episode of Pseudomonas aeruginosa infectionb | 13 (15.7) | 4 (22.2) | 0.497 |
| Inappropriate initial antimicrobial treatment | 37 (44.6) | 9 (50) | 0.795 |
| Definitive inadequate antimicrobial treatment | 3 (3.6) | 2 (11.1) | 0.216 |
| Time to initiate adequate antibiotic therapy (days)a | 1 (0–10) | 0 (0–7) | 0.908 |
| Shock | 30 (36.1) | 5 (27.8) | 0.592 |
| 7-day mortality | 6 (7.2) | 2 (11.1) | 0.630 |
| 30-day mortality | 19 (22.9) | 2 (11.1) | 0.348 |
ICU, intensive care unit; MDR-GNB, multi-drug resistant-gram-negative bacteria.
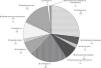
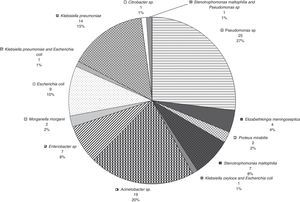
Fig. 1 provides the frequency of each pathogen in the patients with 7-day mortality. The most frequently occurring pathogens in these patients were A. baumanii (37.5%) and Pseudomonas (25.0%). In this group, the bacteria were isolated from the sites as follows: 3 (37.5%) from a combination of tracheal aspirate and blood cultures, 3 (37.5%) from blood cultures, 1 (12.5%) from tracheal aspirate culture, and 1 (12.5%) from a combination of catheter tip, tracheal aspirate, and blood cultures. There was concordance between the isolate microorganisms when the GNB were isolated from a combination of different culture sites.
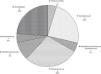
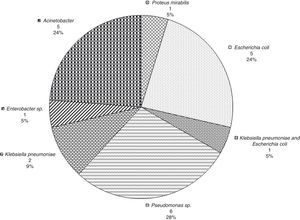
Fig. 2 provides the frequency of each pathogen in the patients who survived for at least 7 days. In these patients, the most frequent sites of bacterial isolation were tracheal aspirate (n=38, 40.9%), blood culture (n=15, 16.1%), and urine culture (n=14, 15.1%).
The most frequently occurring underlying diseases in the patients with 7-day mortality were rhabdomyosarcoma (25%), acute lymphoblastic leukemia (12.5%), acute myeloid leukemia (12.5%), osteosarcoma (12.5%), central nervous system tumor (12.5%), retinoblastoma (12.5%), and non-Hodgkin's lymphoma (12.5%). Only one patient in this group had undergone bone marrow transplantation. Regarding cancer status, 50.0% of the patients were recently diagnosed, 37.5% had experienced disease progression or relapse, and 12.5% had controlled disease.
Among patients who survived seven days after GNB infection, the most frequently occurring diseases were central nervous system tumor (38.7%), neuroblastoma (14%), and non-Hodgkin's lymphoma (9.7%). Regarding cancer status, 50.5% of the patients were recently diagnosed, 29.0% had experienced disease progression or relapse, and 20.4% had controlled disease. None of the patients in this group had undergone bone marrow transplantation.
Among patients with 7-day mortality, there were two (25.0%) episodes of infection at admission (acquired at home), three (37.5%) episodes of healthcare-associated infections acquired at the ward, and three (37.5%) episodes of healthcare-associated infection acquired at the pediatric ICU. Of patients who survived seven days after GNB infection, there were 17 (18.3%) episodes of infection at admission (acquired at home), 19 (20.4%) episodes of healthcare-associated infection acquired at the ward, and 57 (61.3%) episodes of healthcare-associated infection acquired at the pediatric ICU.
Table 2 provides the univariate and multivariate logistic regression risk factor analyses for the 7-day mortality. Shock, neutropenia, antibiotic duration ≤3 days, and definitive inappropriate antibiotic use were associated with 7-day mortality in univariate analyses. Shock (p=0.029) and antibiotic duration ≤3 days (p=0.003) were variables that remained significantly associated with 7-day mortality in the multivariate logistic regression analysis.
Demographic and clinical variables related to 7-day mortality in patients with Gram-negative bacterial infections in a pediatric intensive care unit (ICU), analyzed using univariate and multivariate logistic regression.
| Univariate logistic regression analysis | Multivariate logistic regression analysis | |||||||
|---|---|---|---|---|---|---|---|---|
| Survivorsn=93n (%) | Non-survivorsn=8n (%) | OR | 95% CI | p value | OR | 95% CI | p value | |
| Gender (boys) | 46 (49.5) | 6 (75) | 3.065 | 0.588–15.979 | 0.184 | – | – | – |
| Age (years)a | 6 (0.2–17) | 13 (1–18) | 1.149 | 0.994–1.328 | 0.060 | – | – | – |
| Hematologic diseases | 21 (22.6) | 3 (37.5) | 2.057 | 0.454–9.327 | 0.350 | – | – | – |
| Cancer status (controlled) | 19 (20.4) | 1 (12.5) | 0.556 | 0.064–4.801 | 0.594 | – | – | – |
| Previous antibiotic therapyb | 82 (88.2) | 7 (87.5) | 0.939 | 0.105–8.372 | 0.955 | – | – | – |
| Central venous catheter | 86 (92.5) | 7 (87.5) | 0.570 | 0.061–5.312 | 0.621 | – | – | – |
| Use of corticosteroidsb | 84 (90.3) | 8 (100) | 1.539 | 0.000 | 0.999 | – | – | – |
| Neutropeniac | 22 (23.7) | 5 (62.5) | 5.379 | 1.189– 24.327 | 0.029 | – | – | 0.500 |
| Neutropenia duration ≥3 days | 15 (16.1) | 3 (37.5) | 3.120 | 0.673–14.471 | 0.146 | – | – | – |
| Healthcare-associated infection (from ward and pediatric ICU) | 76 (81.7) | 6 (75.0) | 0.671 | 0.125–3.617 | 0.643 | – | – | – |
| Length of stay before ICU admissiona | 2 (0–89) | 1 (0–7) | 0.926 | 0.795–1.078 | 0.320 | – | – | – |
| Length of ICU stay >3 days | 47 (50.5) | 3 (37.5) | 1.703 | 0.385–7.541 | 0.492 | – | – | – |
| Chemotherapy/radiation therapyb | 65 (69.9) | 7 (87.5) | 3.015 | 0.354– 25.671 | 0.312 | – | – | – |
| Previous episode of Pseudomonas aeruginosa infectionb | 16 (17.2) | 1 (12.5) | 0.688 | 0.79–5.982 | 0.734 | – | – | – |
| Colonization by MDR-GNB | 22/77 (28.6) | 3/6 (50) | 1.091 | 0.163–7.305 | 0.929 | – | – | – |
| Inappropriate initial antimicrobial treatment | 52 (55.9) | 3 (37.5) | 0.473 | 0.107–2.096 | 0.324 | – | – | – |
| Time to initiate adequate antibiotic therapy (days)a | 1 (0–10) | 0 (0–4) | 0.704 | 0.347–1.431 | 0.333 | – | – | – |
| Definitive inappropriate antibiotic treatment | 3 (3.2) | 2 (25) | 10.000 | 1.393–71.766 | 0.022 | – | – | 0.457 |
| Antibiotic duration ≤3 days | 3 (3.2) | 4 (50) | 30 | 4.954–181.686 | <0.001 | 21.328 | 2.834–160.536 | 0.003 |
| Shock | 28 (30.1) | 7 (87.5) | 16.250 | 1.909–138.340 | 0.011 | 12.397 | 1.291–119.016 | 0.029 |
| Infection by MDR-GNB | 42 (45.2) | 5 (62.5) | 2.024 | 0.457–8.966 | 0.353 | – | – | – |
CI, confidence interval; ICU, intensive care unit; MDR-GNB, multidrug-resistant gram-negative bacteria; OR, odds ratio.
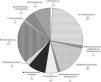
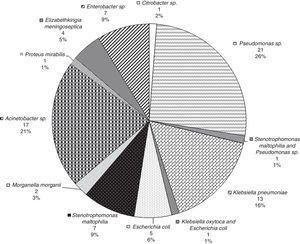
Fig. 3 provides the frequency of each pathogen in patients with 30-day mortality. The most frequently occurring pathogens in these patients were Pseudomonas (28.6%), E. coli (23.8%), and Acinetobacter (23.8%). In this group, the most frequent sites of bacterial isolation were tracheal aspirate (n=5, 23.8%), blood culture (n=5, 23.8%), combination of tracheal aspirate and blood culture (n=3, 14.3%) and urine culture (n=3, 14.3%).
The most frequently occurring underlying diseases in the patients with 30-day mortality were central nervous system tumor (28.6%), acute lymphoblastic leukemia (14.3%), acute myeloid leukemia (9.5%), rhabdomyosarcoma (9.5%), primitive neuroectodermal tumor (9.5%), retinoblastoma (9.5%), non-Hodgkin's lymphoma (9.5%), neuroblastoma (4.8%), and osteosarcoma (4.5%). Only one patient in this group had undergone bone marrow transplantation. Regarding cancer status, 47.6% of the patients were recently diagnosed, 47.6% had experienced disease progression or relapse, and 4.8% had controlled disease.
In the patients who survived 30 days after GNB infection, the most frequently occurring underlying diseases were central nervous system tumor (38.7%), neuroblastoma (15.0%), and non-Hodgkin's lymphoma (10.0%). Regarding cancer status, 51.3% of the patients were recently diagnosed, 25.0% had experienced disease progression or relapse, and 23.8% had controlled disease. None of the patients in this group had undergone bone marrow transplantation.
In the patients with 30-day mortality, there were five (23.8%) episodes of infection at admission (acquired at home), 6 (28.6%) episodes of healthcare-associated infection acquired at the ward, and 10 (47.6%) episodes of healthcare-associated infection acquired at the pediatric ICU. In the patients who survived 30 days after GNB infection, there were 14 (17.5%) episodes of infection at admission (acquired at home), 16 (20.0%) episodes of healthcare-associated infection acquired at the ward, and 50 (62.5%) episodes of healthcare-associated infection acquired at the pediatric ICU.
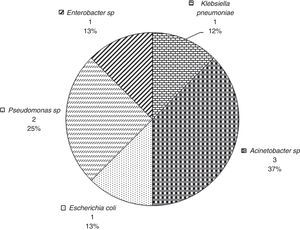
Fig. 4 provides the frequency of each underlying diseases among patients who survived 30 days. In these patients, the most frequent sites of bacterial isolation were tracheal aspirate (n=34, 42.5%), blood culture (n=13, 16.3%), and urine culture (n=11, 13.8%).
The results of the univariate and multivariate logistic regression analyses are provided in Table 3. Only shock (p=0.004) and colonization by MDR-GNB (p=0.016) were significantly associated with 30-day mortality in the multivariate logistic regression.
Demographic and clinical variables related to 30-day mortality in patients with Gram-negative bacterial infections in a pediatric intensive care unit (ICU) analyzed using univariate and multivariate logistic regression.
| Univariate logistic regression analysis | Multivariate logistic regression analysis | |||||||
|---|---|---|---|---|---|---|---|---|
| Survivorsn=80n (%) | Non-survivorsn=21n (%) | OR | 95% CI | p value | OR | 95% CI | p value | |
| Gender (boys) | 43 (53.8) | 9 (42.9) | 0.645 | 0.245–1.702 | 0.376 | – | – | – |
| Age (years)a | 6 (0.2–17) | 11 (0.5–18) | 1.039 | 0.954–1.131 | 0.377 | – | – | – |
| Hematologic diseases | 17 (21.3) | 7 (33.3) | 1.853 | 0.646–5.314 | 0.251 | – | – | – |
| Cancer status (controlled) | 19(23. 8) | 1 (4.8) | 0.161 | 0.020–1.276 | 0.084 | – | – | – |
| Previous antibiotic therapyb | 71 (88.8) | 18 (85.7) | 0.761 | 0.187–3.101 | 0.703 | – | – | – |
| Central venous catheter | 73 (91.3) | 20 (95.2) | 1.918 | 0.223–16.512 | 0.553 | – | – | – |
| Use of corticosteroidsb | 71 (88.8) | 21 (100) | 4.778 | 0.000 | 0.999 | – | – | – |
| Neutropeniac | 20 (25) | 7 (33.3) | 1.5 | 0.531–4.239 | 0.444 | – | – | – |
| Neutropenia duration ≥3 days | 13 (16.3) | 5 (23.8) | 1.611 | 0.502–5.172 | 0.423 | – | – | – |
| Healthcare-associated infection (from the ward and pediatric ICU) | 66 (82.5) | 16 (76.2) | 0.679 | 0.213–2.161 | 0.512 | – | – | – |
| Length of stay before ICU admissiona | 1.5 (0–89) | 2 (0–36) | 0.983 | 0.941–1.027 | 0.456 | – | – | – |
| Length of ICU stay >3 days | 43 (53.8) | 7 (33.3) | 2.324 | 0.848–6.370 | 0.101 | – | – | – |
| Chemotherapy/radiation therapyb | 54 (67.5) | 18 (85.7) | 2.889 | 0.780–10.693 | 0.112 | – | – | – |
| Previous episode of Pseudomonas aeruginosa infectionb | 15 (18.8) | 2 (9.5) | 0.456 | 0.096–2.174 | 0.324 | – | – | – |
| Colonization by MDR-GNB | 14/64 (21.8) | 11/19 (57.9) | 6.286 | 1.185–33.346 | 0.031 | 12.002 | 1.578–91.286 | 0.016 |
| Inappropriate initial antibiotic | 43 (53.8) | 12 (57.1) | 1.147 | 0.435–3.025 | 0.781 | – | – | – |
| Time to initiate adequate antibiotic therapy (days)a | 1 (0–10) | 1 (0–4) | 0.955 | 0.735–1.242 | 0.733 | – | – | – |
| Definitive inappropriate antibiotic treatment | 3 (3.8) | 2 (9.5) | 2.702 | 0.421–17.326 | 0.295 | – | – | – |
| Antibiotic duration ≤3 days | 3 (3.8) | 4 (19) | 6.039 | 1.236–29.509 | 0.026 | 4.758 | 0.764–29.645 | 0.095 |
| Shock | 22 (27.5) | 13 (61.9) | 4.284 | 1.563–11.742 | 0.005 | 6.174 | 1.760–21.664 | 0.004 |
| Infection by MDR-GNB | 35 (43.8) | 12 (57.1) | 1.714 | 0.649–4.525 | 0.276 | – | – | – |
CI, confidence interval; ICU, intensive care unit; MDR-GNB, multidrug-resistant gram-negative bacteria; OR, odds ratio.
Research has previously focused on adult patients, while the sample of the present study included critically ill pediatric patients with cancer and GNB infection, a specific and vulnerable population of which there is little information available, in a pediatric ICU that specializes in the management of patients with cancer and severe complications. Shock emerged as an important predictor of both 7- and 30-day mortality. Furthermore, appropriate antibiotic duration ≤3 days was associated with 7-day mortality, whereas colonization by MDR-GNB was associated with 30-day mortality. At the same time, cancer status was not associated with mortality, potentially owing to the exclusion of palliative care patients from ICU admission. The identification of risk factors for mortality in these patients with GNB infection may assist with the prevention of future infections and planning of appropriate management strategies.
This is particularly important given that sepsis continues to be a common complication in patients with cancer and is responsible for almost half of ICU admissions in nonscheduled surgical patients.3 Oncologic patients represent a considerable proportion of septic patients with up to one in six severely septic patients having malignancies; these patients have a 30% higher risk of death when compared with general patients.3 Children with multiple organ dysfunction syndrome and sepsis are also at a greater risk of mortality than those without sepsis.4
The impact of appropriate empirical antimicrobial therapy has not been clearly established. In the current study, a significant association was not found between mortality and initial appropriate antimicrobial treatment or the time to initiate adequate antibiotic therapy. These results support those reported in previous studies indicating that antibiotic resistance5,6 and initial appropriate antimicrobial treatment7 may have limited impact on mortality.
The relationship between the timeliness of adequate therapy and prognosis is complex. Physicians often prescribe early extended-spectrum antimicrobial therapy to patients that present with severe clinical conditions;5 the clinical outcome is influenced by disease severity and degree of virulence,3 and patients with serious underlying disease and septic shock are less likely to tolerate delays in the administration of effective antibiotics. In contrast, antimicrobial therapy is often delayed in patients who do not have organ dysfunction or for whom there is a delay in a positive blood culture.5 In adults with cancer and Stenotrophomonas maltophilia infection, every additional day of adequate antibiotic therapy has been demonstrated to decrease the risk of mortality by 1.36,7 while a retrospective cohort study suggested that every hour of delay in effective antibiotic administration from the second hour after the onset of persistent or recurrent hypotension (septic shock) significantly increases hospital mortality.8 Moreover, shock has been associated with mortality in a number of studies.3,5,9,10 Therefore, the delay of appropriate antibiotics confers a greater risk of mortality in patients with shock and influences prognosis. The results of the current study, in addition to those of other studies, support the significance of disease severity on patient outcomes. The impact of resistant GNB infection on mortality also depends on the severity of the underlying disease, and the nature and severity of organ failure are major determinants of outcome in critically ill patients with cancer.5 In the present study, the severity of the underlying disease may explain the observed association between a short duration (≤3 days) of antibiotic administration and 7-day mortality. Finally, patients with early mortality never receive antimicrobial therapy.5
However, it has also been shown that a 2–3-day delay in adequate antibiotic therapy has little effect on patient mortality, potentially related to the original focus of the infection.11 For example, poorer outcomes have been observed in patients with non-urinary tract infections, primarily pneumonia and abdominal infections.3 In the present study, pneumonia (38.6%, n=39/101) and bloodstream infections (17.8%, n=18/101) accounted for over half of the infections (56.4%), and these infections are associated with poor prognoses. However, in the current study, the delay of appropriate antibiotics had a limited impact on mortality, regardless of the focus of infection.
The relationship between inappropriate initial empiric antibiotic therapy and mortality may also be affected by several different factors. First, inappropriate antibiotics may have some activity in vivo. Cephalosporin has been associated with treatment failure, but can be effective for Gram negative producing ESBL infections with low minimum inhibitory concentrations,12 with mortality occurring in only 16.6% of patients with serious ESBL-producing GNB infections with cephalosporin susceptibility at a minimum inhibitory concentration of 2–8mcg/mL that were treated with cephalosporin.13 Piperacillin and tazobactam were no more effective than carbapenem in a recent analysis of six prospective cohorts of patients with bacteremia caused by ESBL-producing E. coli.9 Therefore, the use of inappropriate antibiotics may have some effect, even when followed by appropriate antibiotic therapy, and a short delay (e.g., up to two days) in adjusting the definitive antibiotic therapy may not impact mortality if the antibiotic therapy is adjusted according to the results of susceptibility testing.10,11
Furthermore, many issues may influence the efficacy of antibiotics. For example, in vitro success may not translate to in vivo effectiveness due to inadequate dosing, variable pharmacokinetics, or variable penetration into affected tissues, such as the lungs or abscesses. Non-removal of a septic focus, such as an infected intravascular catheter or an intra-abdominal abscess, may compromise cure rates. In addition, an increasingly recognized variable that affects antibiotic efficacy is the mode of administration. The inappropriate use of antibiotics may render a theoretically effective antibiotic ineffective.14
The increasing prevalence of ESBL carriage on ICU admission raises important questions about empiric therapy policies in patients presenting with infection, which may include the use of a carbapenem as first-line therapy.15 Since the emergence of antibiotic resistance is associated with widespread broad-spectrum antibiotic use, the availability of effective treatments becomes limited without the cautious use of carbapenems.15 Owing to the increasing difficulty in choosing initial empirical antibiotics against possible MDR-GNB, clinical interventions for infection control may play an important role in decreasing the prevalence of MDR-GNB, and thereby break the vicious cycle.16 Reducing the incidence of MDR-GNB with multifaceted interventions may be crucial in improving the outcomes related to ICU-acquired infections in developing countries.16
In severely ill hospitalized patients, the normal flora of the bowel can be affected. Under these conditions, patients are predisposed to persistent colonization by exogenous pathogens that cause nosocomial epidemics,17 and a major risk factor for nosocomial infection is prior colonization.18,19 The proportion of positive surveillance cultures increases with a longer hospital stay; therefore, it could be expected that overt colonization would precede bacteremia that occurs following a prolonged ICU stay.20 It has been previously demonstrated that colonization by MDR-GNB is associated with poor clinical outcomes in adults,21 and the current study indicates that MDR-GNB might be associated with longer-term (30-day) mortality. It is unknown whether higher mortality among colonized patients with a prolonged ICU stay is related to more severe injuries, a greater number of interventions, or more severe underlying illnesses. In fact, it is possible that isolation itself contributes to poor clinical performance.21 However, the awareness of MDR-GNB colonization can lead to more appropriate antimicrobial drug use, improve patient outcomes, and decrease the emergence of antimicrobial drug resistance.21
Limitations of the current study include its retrospective design and small sample size. Despite these, significant differences were observed.
In conclusion, a major risk factor for mortality in pediatric oncologic patients with GNB infection is shock. Consequently, it is important to provide aggressive therapy.
Conflicts of interestThe authors declare no conflicts of interest.