The purpose of this study was to identify measles virus in Shanghai in 2012 and study the genotype trend of measles virus epidemic strains during 2000–2012.
MethodsNose and throat swab specimens were collected from 34 suspected measles cases in Shanghai. Measles virus was isolated using Vero-SLAM cells (African green monkey kidney cells/lymphoid signal activating factor-transfected African green monkey kidney cells). The 450bp of C terminus of the N gene and the entire hemagglutinin gene sequence was amplified using RT-PCR. Phylogenetic analysis was performed by comparing the seven measles strains in Shanghai with the reference strains for H1a, H1b and D8 genotypes, as well as the Chinese measles virus vaccine strain.
ResultsSeven measles viruses strains were isolated from the 34 throat swap specimens. Six strains were genotype H1a, which is the predominant strain in China and one strain was genotype D8, which is the first imported strain since 2000. All these seven strains maintained most of the glycosylation sites except subtype H1a, which lost one glycosylation site.
ConclusionSince 2000, measles virus strains in Shanghai are consistent with measles virus from other provinces in China with H1a being the predominant genotype. This study is also the first report of genotype D8 strain in Shanghai. All strains maintained their glycosylation sites except H1a that lost one glycosylation site. These strains could still be neutralized by the Chinese measles vaccine. We suggest that Shanghai Center for Disease Control laboratories should strengthen their approaches to monitor measles cases to prevent further spread of imported strains.
Measles is a leading cause of childhood mortality in developing countries. Although an effective measles vaccine has been available for more than 40 years and greatly reduced measles outbreak globally, risk for illness and death from measles still exist in countries with poor routine vaccination, and measles outbreaks can still be a threat in these countries.1,2 Measles is caused by infection of measles virus (MVs), which is an enveloped virus of the Morbillivirus genus in the Paramyxoviridae family. Since Shanghai carried out the measles surveillance in 2000, 10,684 measles cases were reported and 488 strains of MVs were monitored until 2012. In 2012, 592 measles cases were reported and 248 strains of MVs were monitored in Shanghai. Although MVs is serologically monotypic, genetic characterization of this virus has identified eight classes (A–H), which can be subdivided into 24 genotypes.3,4
The distribution of each genotype is chronically and geographically patterned. For example, genotype A has circulated in the United States, United Kingdom, Russia, China, and Argentina during the last 20 years.5 Group B and C viruses were mostly isolated in Japan, Philippines, Micronesia6 and South Africa.7 Groups D and E viruses appear to be circulating widely in many countries in Western Europe, particularly Germany, Spain, and United Kingdom8; Group F viruses have been isolated in the African countries.9 Group G viruses were first isolated in Montreal, Canada, in 1988 and have been predominantly identified in Indonesia and Malaysia recently.10 Group H viruses form a highly distinct group isolated in four provinces within China during the early 1990s11 and had then been detected in Korea and Japan.12–14 MVs surveillance in China since 1993 revealed that the H1 genotype is the endemic dominant strain, which has H1a, H1b and H1c subtypes, with H1a being the dominant subtype circulating in China.11,15–19
To assist the goal of globally eliminating measles, it is important to carry out molecular epidemiology investigation besides classic epidemiology studies. Molecular epidemiology can support classical epidemiology by identifying transmission pathways and confirming whether the isolated virus genotype is consistent with the predominant genotype circulating in the country or whether it is an imported strain, and region from which the case was imported.20,21 The present study performed detailed genetic characterization on seven measles strains isolated in Shanghai, tempting to trace the source of an imported strain and eventually give suggestions on how to perform genetic surveillance on MVs in China.
Materials and methodsSample preparationNose and throat swab specimens were collected from 34 suspected measles cases in Hongkou District of Shanghai between March 1, 2012 and November 1, 2012. Specimens were kept in 2mL MEM media and stored at 4°C to maintain viral activity. All specimens were sent to the Shanghai Municipal Center for Disease Control and Prevention within 24h for molecular analysis. The specimens were treated with antibiotics (penicillin and streptomycin) overnight and centrifuged at 2000rpm for 5min at 4°C to remove precipitation. The supernatant was preserved at −80°C for further experiments.
Culture of Vero cellsThe Vero and Vero-SLMA cells were allowed to quickly thaw into T25 flasks. Cells were cultured in a humidified air incubator at 37°C with 5% CO2, changing media every 2 days. Once cells reached confluency, cells were digested with 1× trypsin–EDTA at 37°C for 5min. Then, cells were seeded at a density of 5×105cells/mL into 24 well plates and incubated at 37°C in a humidified 5% CO2 incubator until forming a confluent monolayer.
Isolation of MVsVero and Vero-SLMA cells in 24 well plates were infected with 0.2–0.3mL/well of the viral supernatant. Each viral sample was inoculated into three wells and each plate contained three control wells, which were inoculated with 0.2–0.3mL MEM media. After 1h of incubation at 37° C, 1mL of fresh MEM media were added into each well to replace virus-containing media. The cells were then cultured for 7–10 days to induce cytopathogenic effect (CPE). China measles vaccine strain S191 was used as positive control strain. Cells were observed 24h, 48h and 72h after inoculation. CPE+ cells were grown for five passages. CPE− strains were discarded after three passages if they remain CPE−.
Pathological diagnosis of MVsThe MVs were identified using indirect fluorescence assay. Once the adherent cells on 24 well plates showed CPE++ – +++, the cells were scraped and plated onto 12 well slides. Two replicates were examined for each sample including control cells. Samples were allowed to air dry and then fixed with cold acetone–methanol (1:1, v/v) solution. Assays were performed according to the manufacturer's instructions of immunofluorescence antibody (IFA) assay kit and peripheral fluorescent signal was detected by fluorescence microscopy for IFA+.
Viral RNA extraction and RT-PCRThe second passages of CPE+ cells were collected and RNA was extracted using TIANamp virus RNA Kit (TianGen biotech, DP315-R) according to the manufacturer's instruction. The primers were designed according to the entire hemagglutinin (HA) gene and the 450bp C terminus region of the nucleocapsid (N) gene using primer 5.0 software. The primers were synthesized by Shanghai Biological Engineering Technology Co., Ltd. and these sequences of the primers are listed in Table 1.
Primers to amplify the C terminus 450bp of the measles virus N gene.
| Gene | Primer | Position | Sequence (5′–3′) | Fragment (bp) |
|---|---|---|---|---|
| N | N-P1 | 1198–1219 | TAGGGCAAGAGATGGTAAGGAG | 568 |
| N-P2 | 1745–1765 | TGTGTGGACTGGTTCCTAAG | ||
| HA | H1-P1 | 7244–7265 | AAAACTTAGGGTGCAAGATCAT | 845 |
| H1-P2 | 8070–8089 | TGTCATATGGAACACCGGAG | ||
| H2-P1 | 7904–7925 | CGAGGTTACAATGTGTCATCTA | 677 | |
| H2-P2 | 8560–8580 | TGTGTGATCAATGGCCCGAAT | ||
| H3-P1 | 8488–8507 | GATTCCTTCATACGGGGTCT | 730 | |
| H3-P2 | 9195–9217 | GACCCTACGTTTTTCTTAATTCT | ||
RT-PCR was performed using one step RT-PCR kit (Tiangen Biotech). The one step RT-PCR was carried out in a 50μL reaction volume containing 2μL template RNA, 5μL 10× reaction buffer, 2μL dNTP Mix (10mM each), 10μL 5× RT-PCR enhancer, 0.5μL RNasin (40U/μL), 1μL HotmasterTaq polymerase (2.5U/μL), 0.5μL Quant RTase (for one step), 3μL upstream primer (10μM), 3μL downstream primer (10μM), and 23μL nuclease free water. The reaction mixture was amplified at the following conditions: reverse transcription at 50°C for 30min, initial denaturation at 94°C for 2min, followed by 40 cycles at 94°C for 0.5min, 56–61°C for 1min, 65° C for 2min, and a final step at 65°C for 10min.
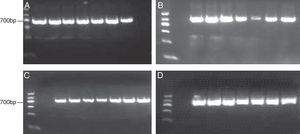
DNA sequencing and genetic characterizationAmplification products were separated by electrophoresis using 1.5% agarose gel containing 0.5μg/mL ethidium bromide, and imaged on Gel Doc™ XR System (Bio-Rad, Hercules, CA). PCR products were purified and sequenced on an ABI 3730XL automated sequencer in Maipu biotech, Shanghai. Sequencing results were analyzed by Chromas software.
The isolated virus strains were characterized according to the World Health Organization (WHO) recommended “MVs genotype identification standard method”.22 Sequences of 23 MVs representative strains and the sequence of Chinese measles vaccine strain, S191 were downloaded from GenBank (Table S1). Target strain sequence and existing sequences in 2000–2012 in Shanghai were analyzed using the Molecular Evolutionary Genetics Analyses (MEGA) software version 4.0.23 The reliability of the analyses was estimated using 1000 bootstrap replicates.
Supplementary Table S1 related to this article can be found, in the online version, at http://dx.doi.org/10.1016/j.bjid.2014.05.018.
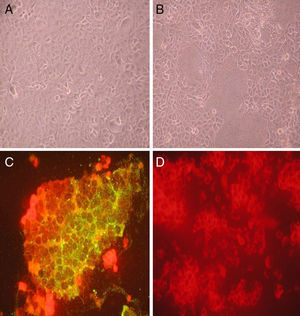
ResultsVirus isolation and pathological analysisThirty-four suspected measles cases were reported in 2012, and 27 cases were confirmed in the research lab and one case was confirmed by clinical diagnosis. The other six cases (rubella or other cases) were excluded for this study. Throat swab specimens were taken from 34 patients and seven of them showed CPE+ after infecting Vero-SLMA cells (Fig. 1A and B). The CPE+ cells showed positive peripheral fluorescent signal by IFA, confirming that the isolated virus were MVs (Fig. 1C and D). The epidemiological information of the seven patients is listed in Table 2. According to the WHO systematic nomenclature for MVs, the seven isolated viruses were named and their short names were SHHK2012-01, SHHK2012-02, SHHK2012-03, SHHK2012-04, SHHK2012-05, SHHK2012-06, SHHK2012-07 in this study. RT-PCR showed 568bp for N gene, and 845bp, 677bp, and 730bp for HA gene (Fig. 2), which are all the characteristic bands for MVs.
Isolation and pathological diagnosis of measles virus by cytopathic effect (CPE) and indirect fluorescence assay. Vero-SLAM cells were infected with control cell culture media (A, D) and virus collected from throat swabs (B, C) for 72h. Cells infected with virus collected from throat swabs showed obvious CPE (B) while cells infected with control media were normal (A). Indirect fluorescence assay was performed on cells infected with control virus and throat swab material. Viral infected cells showed peripheral green fluorescence staining (C) which was absent in control cells (D).
Information of the patient infected by the seven measles strains.
| Number | Gender | Age | Occupation | Residence | Onset of disease | Date of diagnosis | Type of diagnosis | Length of residence | Vaccine history | Source of vaccine history | Nomenclature | NCBI accession number | Abbreviations | Genotype |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | M | 26 | Government staff | Local resident | 2012/8/6 | 2012/8/10 | Laboratory diagnosis | >3 months | Unavailable | Parent memory | MVi/shanghai.CHN/30.12/03 | Unregistered | SHHK2012-01 | H1 |
| 2 | M | 26 | Business | Other province | 2012/7/26 | 2012/7/29 | Laboratory diagnosis | >3 months | Unavailable | Parent memory | MVi/shanghai.CHN/30.12/04 | Unregistered | SHHK2012-02 | H1 |
| 3 | M | 36 | Food industry | Local resident | 2012/8/16 | 2012/8/19 | Laboratory diagnosis | >3 months | Unavailable | Parent memory | MVi/shanghai.CHN/32.12/05 | Unregistered | SHHK2012-03 | H1 |
| 4 | F | 36 | Housekeeping or unemployed | Local resident | 2012/8/8 | 2012/8/12 | Laboratory diagnosis | >3 months | Unavailable | Parent memory | MVi/shanghai.CHN/32.12/06 | Unregistered | SHHK2012-04 | H1 |
| 5 | F | 36 | Government staff | Local resident | 2012/7/22 | 2012/7/29 | Laboratory diagnosis | >3 months | Unavailable | Parent memory | MVi/shanghai.CHN/33.12/06 | Unregistered | SHHK2012-05 | H1 |
| 6 | F | 42 | Housekeeping or unemployed | Other province | 2012/8/17 | 2012/8/20 | Laboratory diagnosis | >3 months | Unavailable | Parent memory | MVi/shanghai.CHN/33.12/07 | Unregistered | SHHK2012-06 | H1 |
| 7 | F | 1 | Children | Local resident | 2012/5/23 | 2012/5/29 | Laboratory diagnosis | >3 months | Unvaccinated | Vaccine record | MVi/shanghai.CHN/40.12/01 | Unregistered | SHHK2012-07 | D8 |
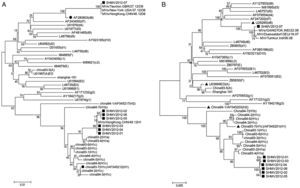
NCBI search revealed 23 MVs reference strains24 and 14 measles subtype reference strains11 in China between 1993 and 1994. Phylogenetic trees were constructed by comparing the C terminal 450bp of N gene (Fig. 3A) and entire HA gene (Fig. 3B) of these reference strains with those of the seven strains isolated in Shanghai in 2012. Among the 14 strains isolated in 1993–1994, 12 belong to the H1 gene family, one (China93-5) belongs to the A gene family, and one (China94-1) belongs to the H2 gene family. Among the 12 H1 strains, China93-2 and China93-4 were H1a subtype; China94-7 is H1b subtype; China93-1, China93-3, China93-6, China93-7, China94-2, China94-3, China 94-4, China 94-5 and China94-6 are H1c subtypes. The China93-7 (AF045212) and China94-1 (AF045217) are standard reference strains for H1 and H2 genotypes, respectively.
Phylogenetic analysis of MVs genotype reference strains and the seven measles strains in Shanghai in 2012 using the C terminal 450bp of N gene (A) and sequence the entire HA gene (B). The dendrogram was constructed using MEGA 4 software. Bootstrap confidence limits were based on 1000 replicates. Numbers at the nodes indicate bootstrapping values. Reference strains of each genotype and subtype are indicated by black triangles. Strains isolated in Shanghai in 2012 are indicated with black squares. D8 and H1c reference strains are indicated by black circles.
Analysis on the N gene sequence indicated that the seven strains isolated in 2012 are not identical. The gene sequences of three strains including SHHK2012-01, SHHK2012-03 and SHHK2012-04 in the same group are identical to that of MVs/HongKong.CHN/49.12 (KC417295.1). The gene sequences of other three strains, including SHHK2012-02, SHHK2012-05 and SHHK2012-06 in another group, were identical. Only one base pair is different between the gene sequences of the two groups (499/450). The difference between the N gene sequences of these six strains and the H1 reference strain Hunan.CHN/93/7 (china93-7, AF045212) is 2.22–2.45% (440/450–439/450, < 2.5%). The difference is 6.67% (420/450) when compared with the H2 reference strain china94-1 (AF045217) and 7.11–11.33% (418/450–399/450) when compared with the other 21 representative strains (Table S1). Therefore, according to the WHO reference sequence for each genotype, these six strains belong to the H1 genotype.24
The sequence of the seventh strain SHHK2012-07 varies from the other six strains by 8.89–9.11% (410/450–409/450), suggesting that the seven strains do not belong to the same gene family. However, SHHK2012-07 is identical to strains MVs/New-York.USA/37.12 [D8] (gb|KC492072.1|), MVs/HongKong.CHN/46.12 [D8] (gb|KC288107.1|), and MVs/Taunton.GBR/27.12/[D8] (gb|JX984461.1|) (450/450), which were isolated in 2012 from USA, Hong Kong and England, respectively. The difference between SHHK2012-07 and the D8 genotype reference strain Manchester.UNK/30.94 (AF280803) is only 2.22% (440/450), and the difference is 4.89% (428/450)–10.0% (405/450) when compared to reference strains from other genotypes. Therefore, we conclude that the SHHK2012-07 belongs to the D8 gene family.
The six strains that belong to the H1 gene family were also compared with reference strains of H1 subtypes. The difference between the six strains with H1a reference strains (China93-2, China93-4) is 1.1–1.3% (445/450-444/450), 3.33–3.11% (435/450-436/450) when compared with H1b subtype (China94-7), and 2.22–2.89% (440/450-437/450) when compared with H1c subtype (China93-7). Because the sequence difference between subtypes was less than 2%, we conclude that these six strains belong to the H1a subtype.
In addition to the nucleotide sequence comparison with the N gene, we also evaluated the differences in entire HA gene sequences of the seven new isolated strains and of the reference strains. Analysis on HA gene sequences demonstrated that there was only 0.1% (1852/1854) variation among the six H1 genotype strains. The difference is 2.10% (1815/1854) when compared with China93-7 (Hunan.CHN/93/7, AF045201), 3.13% (1796/1854) when compared to H2 reference strain China94-1 (Beijing.CHN/94/1, AF045203), and 5.0–7.0% when compared with the other 21 strains (Table S1).
SHHK2012-07 differs with the other six strains by 6.58–6.63% (1732/1854-1731/1854), by 0.86% (1838/1854) when compared to D8 reference strain Manchester.UNK/30.94 (U29285), and by 2.21% (1813/1854)-5.88% (1745/1854) when compared to other 22 D8 strains.
The six H1 strains belong to the same branch with the 12 H1 subtypes from 1993 to 1994. Phylogenetic analysis indicated that these six strains vary by 1.24–1.18% (1831/1854-1832/1854) when compared with H1a reference strains (China93-2, China93-4), by 2.75% (1803/1854) when compared to H1b subtype (China94-7) and by 2.26–1.89% (1812/1854-1819/1854) when compared to H1c strain (China93-7). Therefore, these six strains belong to H1a subtype (Table S1).
Phylogenetic analysis on MVs strain in Shanghai in 2000–2012NCBI search resulted in 104 measles strains during the 2000–2012 in Shanghai. Phylogenetic analysis was performed for these 104 strains and the 14 strains isolated between 1993 and 1994, as well as the Chinese measles vaccine strain S191 (Fig. 4A). Phylogenetic analysis demonstrated that 103 of the 104 strains are genotype H1, while one strain was genotype D8. Among the 103 H1 strains, 97 belong to the H1a subtype. These 97 strains belong to the same cluster with China93-2 and China93-4 with the internal variation of 0–2.22% (440/450). Another six strains together with China94-7 belong to the H1b subtype with an internal variation of 2.45–0.67% (439/450-447/450). Although there are nine H1c subtypes isolated in 1993–1994 with the internal variation of 0–1.33% (444/450-450/450), there was no H1c strain in 2000–2012, suggesting that these strains might have been eliminated in Shanghai. The 97 H1a strains can be further separated into two clusters: 62 strains belong to cluster1 and 35 strains belong to cluster2.
Phylogenetic analysis of the C terminal 450bp of N gene sequence (A) and the entire HA gene sequence (B) of 104 measles strains isolated in Shanghai during 2000–2012, the 14 strains between 1993 and 1994, and the Chinese measles vaccine strain S191. The dendrogram was constructed using MEGA 4 software. Bootstrap confidence limits were based on 1000 replicates. Numbers at the nodes indicate bootstrapping values. Reference strains of each genotype and subtype are indicated by black triangles. Strains isolated in Shanghai in 2012 are indicated with black squares. H1 reference strains are indicated by black circles.
We obtained the HA gene sequences of 65 MVs in China. Using these sequences, we constructed phylogenetic trees for the seven strains in this study, 14 strains in 1993–1994 and the Chinese measles vaccine strain S191 (Fig. 4B).
All 71 H1 genotypes were from 25 provinces in China among which 65 strains were in the same group of China93-4 and China93-2 as H1a subtype with internal variation of 0–2.05% (1816/1854-1854/1854). Another six stains were in the same group of H1b reference strain China94-7 with an internal variation of 0.38–0.92% (1937/1854-1847/1854). The nine strains from 1993 to 1994 were the only cases of H1c subtype with internal variation of 0.05–1.56% (1825/1854-1853/1854), consistent with the conclusion that the H1c subtype might have been eliminated.
The only D8 virus differs from the H1 genotype by 6.63–5.99% (1731/1854-1743/1854) and differs with the Chinese measles vaccine strain S191 by 4.53–5.61% (1770/1854-1750/1854), confirming that this strain does not belong to H1 strain, but rather forms a separate cluster by itself.
In order to determine whether the current MVs vaccine is able to protect against the newly imported D8 strain, we compared the amino acid sequences besides the nucleotide analyses. There was 0–3.73% (594/617) difference within the 71 H1 strains. The variation between these strains with D8 was 6.32–4.53% (578/617-589/617) and 5.51–3.89% (583/618-593/618) when compared with the S191 vaccine strain.
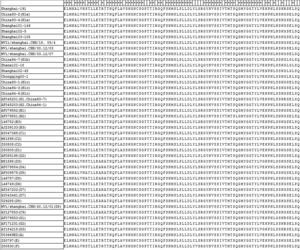
Glycosylation of the HA gene is important for neutralization of the antigenic sites,25 proper folding of the proteins, and has big influence on the antigenicity and immunogenicity of the HA glycoprotein.26,27 In order to understand whether the current Chinese measles vaccine can induce protection against the seven isolated MVs strains, we compared the locations and mutations of the glycosylation sites. The results indicated that the serine (S) 240 of the H1a subtype was mutated to asparagine (N) by a single point mutation of adenine 719 to guanine, which destroyed one glycosylation site, while this site was maintained in H1b, H1c subtype and in genotype D8 (Fig. 5). When we examine the reference strain for the other 21 genotypes, we found that all strains maintained five N glycosylation sites except for genotype D5, B3 and G3. Genotype D5 lost the glycosylation site at 169, while B3 and G3 lost a glycosylation site at 238. Based on the analysis on the glycosylation site, we predict that the Chinese measles vaccine is still able to induce protection against the predominant strains as well as against the 22 imported strains.
The location and mutations of the glycosylation site in measles strains isolated in Shanghai compared to the H1c subtype reference strains and the S191 vaccine strain. The H1 strains isolated in Shanghai lost glycosylation site at 238–240 (N-L-S) whereas the D8 strain maintained this glycosylation site.
Epidemiological surveys show that the D8 strain infected patient was a female child born in Shanghai on January 26, 2012. The patient started to have fever in the morning on September 28, 2012, who was later diagnosed of “suspected measles” at Fudan University Children's Hospital. This case was reported through the Internet on October 2nd at 2:34pm. Throat swab and serum specimens were sent to the laboratory for virus testing. On October 9th, collected serum turned out positive for measles IgM antibody. Shanghai Center for Disease Control (CDC) reported positivity for MVs on November 6th and further examination at Chinese Center for Disease Control confirmed that the MVs was of genotype D8 on December 24th.
The father of the child started presenting with fever in the morning on September 18th, 2012. He was also diagnosed as “suspected measles” by Shanghai Public Health Clinical Center at 14:00 on September 23 and the patient was immediately isolated for treatment. Serological testing turned out positive for measles IgM antibodies on September 24th. The patient was hospitalized until September 28th to collect throat swab specimens. Before the onset of fever, the child patient had never traveled overseas. Her father, however, did two business trips between Shanghai and South Korea 7–21 days before onset of his disease. He could have potentially got in contact with the virus on public transportations (such as airplane or airport shuttles) where he came across many international tourists. The child might have been infected by MVs from her father who had imported the virus on his business trips.
DiscussionMolecular epidemiological surveillance of MVs is an important part of global measles control and elimination, which can be achieved by monitoring the distribution of genotypes in different countries and different regions of the same country. The present study performed detailed molecular characterization on seven measles strains identified in Shanghai during 2000–2012. Through the detailed analysis of the HA and C terminus 450bp of the N gene, we concluded that six of the seven measles strains belong to the H1 genotype and H1a subtype. The remaining strain was of genotype D8. There was no H1c strain in 2000–2012, suggesting that this strain might have been eliminated in Shanghai. This trend is consistent throughout the country.15–19 China started surveillance for MVs in 1993.17 According to recent studies on circulation of MVs strains in China, H1c might have been eliminated because the last cases of H1c subtype occurred in 1993–1994 and there has been no further report of H1c strain. However, H1a and H1b were co-circulating between 1993 and 2005. The circulation of H1b already started to decrease following vaccination in 2005, and only H1a has remained as the predominant strain in China.17 The virus strain isolated in Shanghai is consistent with the geographic and genotypic distributions in other provinces of China, suggesting that the current measles vaccine should be sufficient to protect people in Shanghai and district specific immunization is not necessary at the current stage.
This study is the first report for the occurrence of a D8 strain in Shanghai. D8 MVs is predominantly circulating in Bangladesh, India and Nepal.28,29 There have also been D8 import reports in the USA, UK, Australia, and Central Europe since 2000. Because of the wide distribution of the D8 virus, it is difficult to determine the original source of the D8 strain. In China, there have only been four genotypes of imported MVs cases previously. One H2 genotype was detected in Beijing in 1994, one D9 genotype in Sichuang in 2009, one D4 case from Shanxi, and one D11 case in Yunnan. Following detection of the last case in Yunan a severe measles outbreak has ensued in the subsequent 2 years.30–33 Although there have been several reports on MVs analysis in Shanghai in the past few years,34,35 this is the first time that the MVs epidemic analysis went through a 12-year span and provided important information on MVs control.
Glycosylation of the HA gene, which is important for neutralization of the antigenic sites25 and proper folding of the proteins, has big influence on the antigenicity and immunogenicity of the HA glycoprotein.26 For example, the Edmonston MVs vaccine strain in 1960 contains five N linked glycosylation sites.27 These sites are located at the N terminus 168–170 (NST), 187–189 (NCS), 200–202 (NMS), 215–217 (NVS) and 238–240 (NLS).27,36 Mutations at the glycosylation sites will cause instability and structural change on HA protein and influence its immunogenicity. Analysis on amino acid sequences revealed that most of the glycosylation sites are maintained except for H1a subtype containing one amino acid mutation. Although mutations at the glycosylation sites have big impact on antigenicity,25,27 H1a subtypes and D8 strain both maintained most of the glycosylation sites, suggesting that the current vaccine can still induce protection against the D8 imported strain.
However, the importance of detecting an imported strain cannot be neglected because imported strains caused severe measles outbreak during the past 20 years.37–40 China has committed to eliminate endemic MVs transmission by 2012.41 It is critical to investigate the genotype of circulating MVs in order to control the spread of imported strains.
In conclusion, the present study performed detailed molecular characterization on seven MVs strains in Shanghai in 2012. Six strains belonged to the H1a subtype, which is consistent with the predominant strain in other provinces in China. One strain was genotype D8 and was detected for the first time in China. These strains contain most of the N linked glycosylation sites. Therefore, the current measles vaccines used in China can induce protection against these strains.
Shanghai as an international metropolis serves as one of the major trading ports and gateways for all over the world. The fast economic developments make Shanghai not only a diversified international culture exchange center, but also make the city vulnerable for imported virus strains and make it challenging for diseases control at the same time. With the development of molecular biology techniques, it is entirely feasible to carry out gene sequencing and molecular characterization of MVs surveillance in regional laboratories. This can help strengthen the measles laboratory monitoring network and improve monitoring efficiency.
FundingThis work was supported by grants from: 1. the Excellent Academic Leaders of Shanghai public Health field (No.GWDTR201225). 2. the Shanghai Municipal Health & Family planning Commission (No.20114067).
Conflicts of interestThe authors declare no conflicts of interest.
The authors thank the work team of Department of Immunization programs (District Center for Diseases Control and Prevention of Hongkou, Shanghai, China) for collecting measles swabs samples and epidemiological investigations of cases.