Children with cerebrospinal fluid pleocytosis are frequently treated with parenteral antibiotics, but only a few have bacterial meningitis. Although some clinical prediction rules, such as bacterial meningitis score, are of well-known value, the cerebrospinal fluid white blood cells count can be the initial available information. Our aim was to establish a cutoff point of cerebrospinal fluid white blood cell count that could distinguish bacterial from viral and aseptic meningitis. A retrospective study of children aged 29 days to 17 years who were admitted between January 1st and December 31th, 2009, with cerebrospinal fluid pleocytosis (white blood cell≥7μL−1) was conducted. The cases of traumatic lumbar puncture and of antibiotic treatment before lumbar puncture were excluded. There were 295 patients with cerebrospinal fluid pleocytosis, 60.3% females, medium age 5.0±4.3 years distributed as: 12.2% 1–3 months; 10.5% 3–12 months; 29.8% 12 months to 5 years; 47.5% >5 years. Thirty one children (10.5%) were diagnosed with bacterial meningitis, 156 (52.9%) viral meningitis and 108 (36.6%) aseptic meningitis. Bacterial meningitis was caused by Neisseria meningitidis (48.4%), Streptococcus pneumoniae (32.3%), other Streptococcus species (9.7%), and other agents (9.7%). cerebrospinal fluid white blood cell count was significantly higher in patients with bacterial meningitis (mean, 4839cells/μL) compared to patients with aseptic meningitis (mean, 159cells/μL, p<0.001), with those with aseptic meningitis (mean, 577cells/μL, p<0.001) and with all non-bacterial meningitis cases together (p<0.001). A cutoff value of 321white blood cell/μL showed the best combination of sensitivity (80.6%) and specificity (81.4%) for the diagnosis of bacterial meningitis (area under receiver operating characteristic curve 0.837). Therefore, the value of cerebrospinal fluid white blood cell count was found to be a useful and rapid diagnostic test to distinguish between bacterial and nonbacterial meningitis in children.
Despite the advances in diagnosis and treatment of infectious diseases, meningitis is still considered as an important cause of mortality and morbidity, specially in the pediatric population.1,2 Bacterial meningitis (BM) can cause serious complications and its severity depends not only on the causal microorganism, but also on host immune factors, immunization status, and geographic region.3 The most common etiological agents are Neisseria meningitidis and Streptococcus pneumoniae, the latter being associated with a higher rate of severe and permanent sequelae, and mortality.4,5 The implementation of vaccination programs allowed a remarkable reduction in incidence and mortality of infectious diseases. The incidence of invasive disease by Haemophilus influenzae (Hib) decreased dramatically in populations with high immunization coverage rates.6,7 More recently, meningococcal conjugate type C and pneumococcal vaccines have also contributed to change the epidemiological profile of this disease.8,9
When approaching a child with meningitis it is known that an early introduction of antibiotic treatment assures rapid treatment of children with BM. However, antibiotic therapy results in systematic hospitalization and unnecessary antibiotic administration for children with aseptic or viral meningitis (VM), with the associated morbidity and economic costs. Therefore, distinguishing BM from other types of meningitis in the emergency department could help to limit unnecessary antibiotic use and hospital admissions. Because the consequences of delayed diagnosis of BM can be severe, any proposed diagnostic tool must achieve near 100% sensitivity.10 Some criteria such as Gram staining, bacterial antigen testing of cerebrospinal fluid (CSF) as well as the classic biological markers in the blood (CRP level, white blood cell [WBC] count, and neutrophil count) or CSF (protein level, glucose level, WBC count, and neutrophil count) can be used to help predicting BM. Some scores like the BM score and the Meningitest have a high sensitivity and are proven to be valid when evaluating a child with meningitis.11–13 More recently, some isolated factors14,15 also proved to be good parameters to differentiate bacterial from VM. However, in some institutions, these results can be time consuming, and in some cases are impossible to be obtained. Therefore, our aim was to verify the possibility of using the CSF WBC count in an initial evaluation of BM. The objective of the present study was to establish a cutoff point of CSF WBC count that distinguished bacterial from viral and aseptic meningitis.
MethodsChildren aged 29 days to 17 years, admitted to Centro Hospital São João, Oporto, Portugal, with CSF pleocytosis (considered as a WBC count ≥7μL−1), were enrolled in this retrospective study from January 1st, 2005 to December 31th, 2009. Cases of traumatic lumbar puncture (LP) and those who had received antibiotic treatment before LP were excluded.
The diagnosis of meningitis was based on history, physical examination and CSF laboratory findings. Meningitis was defined as bacterial according to identification of bacterial agents in Gram staining and/or positive bacterial culture. It was defined as viral if the reverse transcriptase polymerase chain reactions were positive, and the bacterial culture was negative. The other cases were considered as aseptic meningitis.
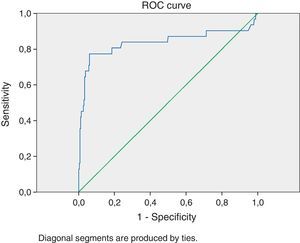
Statistical analysis of data was carried out using the SPSS 18 software. Differences between groups in continuous variables were tested for significance with the student's t test. Differences in frequencies of findings between groups were analyzed by Fischer's exact test. The p-value was considered significant if <0.05. The CSF markers were analyzed to determine specificity and sensitivity of each marker and combinations between them. We used the receiver operating characteristic (ROC) curve to evaluate clinical usefulness of the WBC count. The ROC curve represents the probability of true results in a disease as a function of the probability of false positive results of a test. The area under the curve represents the validity of a test with 1.00 being the highest and 0 the lowest. A classification for accuracy of a diagnostic test considers 0.90–1.00=excellent; 0.80–0.89=good; 0.70–0.79=fair; 0.60–0.69=poor; 0.50–0.59=failure (Fig. 1).
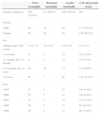
ResultsThe demographic characteristics of patients are summarized in Table 1. When excluding the cases of traumatic LP and those with previous antibiotic treatment (a total of 98) we found 295 patients with CSF pleocytosis. There was a female predominance in all types of meningitis, with 60.3% females in total. The medium age was 5.0±4.3 years distributed as: 12.2% 1–3 months; 10.5% 3–12 months; 29.8% 12 months to 5 years; and 47.5% >5 years. BM was evenly distributed in all age groups, while VM was much more frequent among children >12 months. This difference of age distribution between viral and BM was significant (p<0.05). Thirty one children (10.5%) had BM, 156 (52.9%) VM and 108 (36.6%) AM. The rate of BM was 14.9% in 2005, 26.4% in 2006, 24.7% in 2007, 20.3% in 2008 and 13.6% in 2009. BM was the prevailing type of meningitis in 2007, representing 29% of all bacterial cases.
Demographic features of all groups.
| Viral meningitis | Bacterial meningitis | Aseptic meningitis | CSF pleocytosis (total) | |
|---|---|---|---|---|
| Number of patients | 156 (52.9%) | 31 (10.5%) | 108 (36.6%) | 295 |
| Gender | ||||
| Male | 60 | 12 | 45 | 117 (39.7%) |
| Female | 96 | 19 | 63 | 178 (60.3%) |
| Age | ||||
| Medium age±SD (years) | 5.4±3.9 | 3.6±5.0 | 4.9±4.9 | 5.0±4.3 |
| <3 months | 13 | 5 | 18 | 36 (12.2%) |
| ≥3 months and <12 months | 8 | 9 | 14 | 31 (10.5%) |
| ≥12 months and <5 years | 48 | 10 | 13 | 71 (29.8%) |
| ≥5 years | 87 | 7 | 46 | 140 (47.5%) |
| Year | ||||
| 2005 | 15 | 8 | 21 | 44 (14.9%) |
| 2006 | 59 | 7 | 12 | 78 (26.4%) |
| 2007 | 32 | 9 | 32 | 73 (24.7%) |
| 2008 | 24 | 5 | 31 | 60 (20.3%) |
| 2009 | 26 | 2 | 12 | 40 (13.6%) |
The etiology of meningitis is summarized in Table 2. BM was caused by N. meningitidis (48.4%), S. pneumoniae (32.3%), other Streptococcus species (9.7%), Staphylococcus aureus (3.2%), H. influenzae (3.2%), and Escherichia coli (3.2%). VM was caused by Enterovirus (98.1%), herpes simplex virus type 1 (1.3%), and varicella zoster virus (0.6%).
Agents involved in bacterial and viral meningitis.
| Bacterial meningitis | Viral meningitis | |
|---|---|---|
| Agents identified | Neisseria meningitidis (48.4%), Streptococcus pneumoniae (32.3%), other Streptococcus species (9.7%) and other agents (9.7%) | Enterovirus (98.1%), herpes simplex type 1 virus (1.3%), varicella zoster virus (0.6%) |
When analyzing CSF characteristics (Table 3) WBC count was significantly higher in patients with BM (mean, 4839cells/μL) as compared to patients with VM (mean, 159cells/μL, p<0.001), with those with AM (mean, 577cells/μL, p<0.001) and with both (p<0.001). CSF protein level was also higher in BM than in VM (p<0.01). Since in our hospital the differential counting of the cells with polymorphonuclear leukocyte count is not always performed, this was not subject of analysis.
Table 4 shows the sensitivity and specificity of different values of WBC count for BM patients. The diagnostic cutoff level of 321WBC/μL in CSF maximized was found to have optimum sensitivity (80.6%) and specificity (81.4%), with an area under the ROC curve of 0.837.
DiscussionHospitalization and treatment with broad-spectrum antibiotics in a child with CSF pleocytosis not caused by bacterial agents is frequent and constitute a source of parental stress and increased health costs. On the other hand, failure to promptly diagnose and treat BM can have devastating consequences. The ultimate confirmation of this diagnosis is CSF bacterial culture. However, physicians must make treatment decisions before culture results are available, and they depend on CSF findings to help them do so. Furthermore, a clinical prediction parameter to accurately identify patients at risk of BM is desirable. The search for simple CSF parameter predictor has been a concern of several authors.16,17 Our present study analyzed in a retrospective way the CSF WBC count aiming at establishing a cutoff WBC value to predictive of BM. Several studies showed that the CSF profile alone could not reliably differentiate bacterial from other types of meningitis.18–20 However, we found in our study a very large area under the ROC curve when testing WBC count at the cutoff of 321/μL, as well as high sensitivity and specificity for this parameter, when comparing with similar studies,21,22 and even when compared with other CSF parameters, like protein or glucose levels.23,24 Only 14.7% of our patients with VM had a WBC count in LCR >321μL−1. The fact that this parameter was statistically significant to differentiate BM from both VM and AM came as a surprise to us. Our intention was not to replace scores already studied and well documented, but to try to prove that a single simple parameter could, in an emergency setting, guide a clinical decision. Also, we do not want to downplay the importance of the clinical presentation and the physical examination for diagnosing BM.
ConclusionThe current knowledge showed the existence of very sensitive and specific parameters, including some well-studied scores, used to identify BM. Because these scores differentiate bacterial from nonbacterial meningitis better than a single laboratory value, the current proposal is a multivariable approach. Despite that, the CSF WBC count was also found to be a useful and rapid diagnostic test to distinguish between bacterial and nonbacterial meningitis in children. It can be useful as an initial approach or in situations or places when the time is limited or the resources are scant. A cutoff value of 321WBC/μL has highly sensitive and specific for the diagnosis of BM. As a retrospective study, its limitations are obvious. This study concerns the cases of a tertiary center, where the number of meningitis is probably higher when compared to other hospitals. Thus, it is questionable if this cutoff can be extrapolated to other settings.
Conflict of interestAll authors declare that they have no conflict of interest.