Biofilms formed by Candida albicans, a human pathogen, are known to be resistant to different antifungal agents. Novel strategies to combat the biofilm associated Candida infections like multiple drug therapy are being explored. In this study, potential of chloroquine to be a partner drug in combination with four antifungal agents, namely fluconazole, voriconazole, amphotericin B, and caspofungin, was explored against biofilms of C. albicans. Activity of various concentrations of chloroquine in combination with a particular antifungal drug was analyzed in a checkerboard format. Growth of biofilm in presence of drugs was analyzed by XTT-assay, in terms of relative metabolic activity compared to that of drug free control. Results obtained by XTT-metabolic assay were confirmed by scanning electron microscopy. The interactions between chloroquine and four antifungal drugs were determined by calculating fractional inhibitory concentration indices. Azole resistance in biofilms was reverted significantly (p<0.05) in presence of 250μg/mL of chloroquine, which resulted in inhibition of biofilms at very low concentrations of antifungal drugs. No significant alteration in the sensitivity of biofilms to caspofungin and amphotericin B was evident in combination with chloroquine. This study for the first time indicates that chloroquine potentiates anti-biofilm activity of fluconazole and voriconazole.
Ability of Candida albicans to exist in sessile community form provides tolerance to stress conditions.1 Formation of community growth is initiated by surface induced gene expression.2 Adhesion of cells to tissue surfaces or solid surface of prostheses in the patient's body activates cellular events leading to formation of biofilms.3 Biofilms are drug resistant growth forms which pose a serious threat to immunocompromised patients.4,5 Resistance to various antifungal drugs showed by C. albicans biofilms is considered as a multi-factorial phenomenon involving different cellular mechanisms.6,7 No single antifungal antibiotic was found effective against biofilm related C. albicans infections at low concentrations. Higher concentrations of the antifungal drugs are not advisable because of side effects due to toxicity;8 hence there is a need to devise novel anti-biofilm strategies.9 Combination of drugs which act on different cellular targets simultaneously may offer increased drug efficacy and specificity.10,11 Chloroquine (CQ) has been extensively used against malaria for almost seventy years.12 CQ is believed to target polymerization of ferriprotoporphyrine IX (heme moiety) which creates toxic environment inside plasmodial parasite resulting into cell death.13
Although CQ is used as a first line drug against malaria in many countries like Indonesia, India, Papua New Guinea, Myanmar, Guyana and South America its use has been suspended due to emergence of CQ-resistant strains of Plasmodium falciparum as well as P. vivax.14,15 Emerging resistance to CQ has posed a limitation on its antimalarial use; however, efforts are being done to repurpose this molecule for novel therapeutic activities. For example, CQ has been used as an antiretroviral in clinical trials in HIV infected patients with tumors.16 In a study by Sotelo et al., CQ was found effective in therapeutics against glioblastoma in HIV patients.17 CQ was found to possess antifungal activities against fungal pathogens Cryptococcus neoformans and Histoplasma capsulatum.18,19 Surprisingly, its effects on the most common fungal pathogen C. albicans have not been studied. This study discusses effects of CQ on C. albicans growth in planktonic and biofilm forms. Efficacy of CQ in combination with four important antifungal drugs, namely fluconazole, voriconazole, amphotericin B, and caspofungin, is tested against planktonic cells, biofilm development as well as mature biofilms of C. albicans.
Materials and methodsCultures and antifungal agentsA standard strain of C. albicans, ATCC 90028, was obtained from the Institute of Microbial Technology (IMTECH), Chandigarh, India. Culture was maintained on yeast peptone dextrose (YPD) agar slants at 4°C (all the media components were purchased from HiMedia laboratories Pvt. Ltd. Mumbai, India). Antifungal agents Fluconazole (FLC) (Forcan, Cipla Ltd., India), Voriconazole (VOR) (Vonaz, United Biotech Pvt. Ltd., India), Caspofungin (CSP) (Cancidas™, Merck & Co. Inc., USA), Amphotericin B (AmB) (Lyka Pharm. Pvt. Ltd., India), and Chloroquine (CQ) (Lariago®, Ipca Laboratories Ltd., India) were obtained from local market.
Medium and culture conditionsActivation of culture was done by inoculating a single colony from YPD agar plate into 50mL YPD broth (yeast extract 1%, peptone 2%, and dextrose 2%) in a 250mL conical flask. The flasks were incubated at 30°C at 100rpm on an orbital shaking incubator for 24h. Cells were harvested by centrifugation at 2000×g and washed thrice with 0.1M phosphate buffer saline (PBS), pH 7.4. Final cell number was adjusted to 1×107cells/mL and used for experiments.
Susceptibility of planktonic cells to antifungal drugsThe susceptibility study was carried out by the standard broth micro dilution method as per CLSI guidelines. Various concentrations of drugs were prepared; FLC was in the range 128–0.125μg/mL, while VOR was between 8μg/mL and 0.015μg/mL. Range of concentrations used for CSP and AmB were 4–0.007μg/mL. The concentrations of CQ were in the range 500–0μg/mL. All the drug concentrations were prepared in RPMI-1640 medium pH 7.0 (with l-glutamine, without sodium bicarbonate, buffered with 165mM MOPS; purchased from HiMedia laboratories Pvt. Ltd., Mumbai, India), in the wells of micro plates by double dilution. Wells without drug served as a control. Cells were added to each well to obtain density of 1×103cells/mL. Plates were incubated at 35°C for 48h and absorbance was read at 620nm using a microplate reader (Multiskan EX, Thermo Electron Corp., USA). The lowest concentration of the drugs which caused fifty percentage reduction in the absorbance compared to that of control was considered as minimum inhibitory concentration (MIC).7
Checkerboard format for determination of FICIDilutions of individual drugs and their different combinations were prepared in a checkerboard format. A two dimensional array of serial concentrations of test compounds was used for preparation of dilutions of the drugs. 100μL of the cell suspension was added to each well and the plates were incubated at 35°C. Plates were read spectrophotometrically at 620nm, after 48h of incubation. The FIC Indices were calculated using following equation: ∑FIC=FICA+FICB, where FICA=(MIC of drug A in combination/MIC of drug A alone), FICB=(MIC of drug B in combination/MIC of drug B alone). When the value of ∑FIC≤0.5 it is the synergism, and when ∑FIC>4 it is known as the antagonism. A ∑FIC result of >0.5 but ≤4 is considered as indifference.20,21
Biofilm formationBiofilm formation was carried using in vitro biofilm model.7 Briefly, 100μL of cell suspension (1×107cells/mL in PBS) was added to wells of tissue culture grade polystyrene micro plates. Plates were incubated at 37°C on an orbital shaker for 90min of adhesion phase. All the wells were washed with sterile PBS to remove non-adhered cells. Two hundred μl of RPMI-1640 medium, pH 7.0, containing various concentrations of the drugs was added to each well. The plates were incubated at 37°C at 100rpm in an orbital shaker for 48h. Quantitation of growth was done by using XTT-metabolic assay, where the colored end product was read spectrophotometrically.
Biofilm quantitation by XTT assayBiofilm formation was quantitated using XTT [i.e. 2,3-bis (2-methoxy-4-nitro-sulfophenyl)-2H-tetrazolium-5-carboxanilide] (Sigma–Aldrich, India) reduction assay.7 Briefly, XTT solution was prepared by mixing 1mg/mL XTT salt in PBS and stored at −20°C. Prior to use, menadione solution prepared in acetone (Sigma–Aldrich, India) was added to XTT to a final concentration of 4μM. The wells containing biofilms were washed with PBS to remove non-adhered cells and 100μL of XTT-menadione solution was added to it. The plates were incubated for 5h in dark at 37°C at 100rpm. Intensity of the colored formazan end product was measured at 450nm using a micro plate reader. Wells without biofilms served as a blank.
Microscopic analysis of biofilmsBiofilms were observed under an inverted light microscope (Metzer, India). Photographs were taken by a Labomed microphotography system (Labomed, Mumbai, India). For scanning electron microscopy (SEM), samples were fixed in 2.5% glutaraldehyde in 0.1M phosphate buffer (pH 7.2), for 24h at 4°C. Samples were post-fixed in 2% aqueous solution of osmium tetraoxide for 4h, then dehydrated in a series of graded alcohols and finally dried to a critical drying point with a Critical Point Dryer unit. The samples were mounted over stubs, and gold coating was performed using an automated gold coater (model JOEL JFC-1600) for 3min. Photographs were taken under a scanning electron microscope (model JOEL-JSM 5600).7
ResultsSusceptibility of planktonic growth of C. albicans to various antifungal drugs and their combinations with chloroquinePlanktonic growth of C. albicans was susceptible to different antifungal drugs at varying concentrations. MICs of FLC, VOR, AmB, and CSP were found at 0.5μg/mL, 0.25μg/mL, 0.125μg/mL and 0.25μg/mL, respectively (Table 1). Chloroquine did not show any significant effect on growth of C. albicans and MIC was observed at 1mg/mL. No significant decrease in MICs of two azoles, FLC and VOR could be observed when cells were treated in combination with various concentrations of CQ. FIC indices for combination of 250μg/mL concentration of CQ with azole drugs were found to be >0.5. Similar observations were noted for CQ-AmB interactions indicating no significant reduction in the MIC of AmB. Addition of CQ could not lower down the CSP concentration required to inhibit growth of C. albicans. It was evident with the FIC index of 0.74 for this combination (Table 1).
MICs of five antifungal drugs alone and in combination with 250μg/mL CQ for planktonic and biofilm growth of C. albicans.
| Name of the drug | Minimum inhibitory concentration (in μg/mL) | |||||
|---|---|---|---|---|---|---|
| Planktonic cells | Biofilm development | Mature biofilms | ||||
| Antifungal drug | Antifungal drug+CQ | Antifungal drug | Antifungal drug+CQ | Antifungal drug | Antifungal drug+CQ | |
| FLC | 0.5 | 0.5 | NAa (>128) | 4 | NAa (>128) | 4 |
| VOR | 0.25 | 0.125 | NA (>8) | 0.25 | NA (>8) | 0.25 |
| CSP | 0.125 | 0.062 | 0.25 | 0.25 | 0.5 | 0.25 |
| AmB | 0.125 | 0.062 | 0.5 | 0.125 | 1 | 0.5 |
FLC, fluconazole; VOR, voriconazole; CSP, caspofungin; AmB, amphotericin B; CQ, chloroquine.
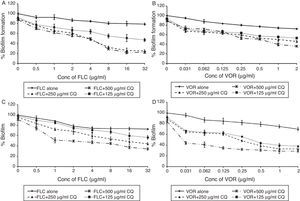
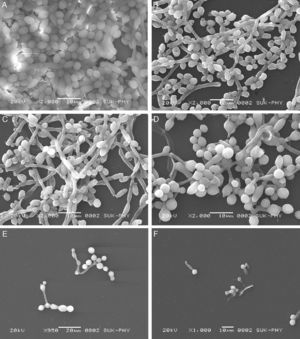
FLC and VOR alone were ineffective against biofilm formation. No significant inhibition of C. albicans biofilm development could be obtained even at high concentrations of FLC and VOR, i.e. 128 and 8μg/mL, respectively. This indicated resistance toward the azole drugs. After combination with CQ, concentrations of FLC and VOR required to inhibit biofilms were lowered down dramatically. Various CQ-FLC combinations were found to effectively inhibit biofilm development in C. albicans at 2–8μg/mL. For example, addition of 250μg/mL of CQ resulted in inhibition of biofilm development at 4μg/mL of FLC. Similarly, CQ-VOR was also effective and MIC of VOR for formation of C. albicans biofilms was achieved at 0.25μg/mL (Fig. 1A and B). FIC indices for CQ-FLC and CQ-VOR combinations against biofilm development were found to be 0.156 and 0.187, respectively, indicating synergistic activity. Results of biofilm inhibition analyzed by XTT metabolic assay were confirmed by light as well as electron microscopy. Control without drug exhibited typical biofilm structure consisting of dense network of filamentous forms and yeast cells attached to the solid surface (Fig. 2A). Although cell density was less, normal biofilms were observed in presence of CQ, FLC and VOR alone (Fig. 2B, C and D, respectively). No biofilm growth was observed in presence of FLC-CQ combination (Fig. 2E). Similarly, VOR-CQ treatment resulted in inhibition of biofilms and only few yeast cells were found adhered to the surface (Fig. 2F). The MIC of AmB for biofilm development was at 0.5μg/mL, which was fourfold more than that of planktonic MIC. When combined with 250μg/mL of CQ the effective AmB concentration was 0.5μg/mL. The FIC index of 1.126 for this combination indicated indifference activity in combination. Similarly, combination of CQ with CSP exhibited no change in the MIC for C. albicans biofilm development (Table 1). FIC index of 0.621 for CQ-CSP was indicative of additive effect.
Activity of FLC and VOR antifungal drugs alone and in combination with CQ, against biofilm development and mature biofilms of C. albicans (ATCC 90028). Percentage of growth was analyzed by comparing relative metabolic activity (RMA) obtained through XTT metabolic assay. (A) FLC-CQ against biofilm development; (B) VOR-CQ against biofilm development; (C) FLC-CQ against mature biofilms; (D) VOR-CQ against mature biofilms.
Scanning electron micrographs of FLC and VOR antifungal drugs alone and in combination with CQ, against biofilm development of C. albicans (ATCC 90028). (A) Control; (B) CQ alone 250μg/mL; (C) FLC alone (128μg/mL); (D) VOR alone (8μg/m); (E) 4μg/mL FLC+250μg/mL CQ; (F) 0.25μg/mL+250μg/mL CQ.
Mature biofilms of C. albicans were highly resistant toward activity of FLC and VOR. MICs were not achieved even at very high concentrations, i.e. 128μg/mL of FLC and 8μg/mL of VOR. When 250μg/mL of CQ was added to the mature biofilms, MIC of FLC was obtained at 4μg/mL. This indicated effective combination, with FIC index of 0.25. Combination of CQ with VOR resulted in inhibition of mature biofilms at concentrations as low as 0.25μg/mL (Fig. 1C and D). The FIC index for CQ-VOR combination was calculated to be 0.187. Although C. albicans biofilms were found sensitive to AmB and CSP, it required high concentrations. When combined with CQ, twofold decrease in the effective concentrations of AmB and CSP was observed (Table 1). The FIC indices for CQ-AmB and CQ-CSP drug combinations were 0.496 and 0.366, which are considered to exert synergistic effect.
DiscussionInfections associated with biofilms of C. albicans is a serious problem in immune-compromised population.1 Novel strategies for prevention and treatment of drug resistant biofilms are required urgently. Various reasons have been proposed to be responsible for the antifungal resistance of C. albicans biofilms.6 Factors like extracellular polymer, overexpression of drug efflux pumps may contribute to the drug resistance.22 Combination of drugs with different mode of action may inhibit multiple cellular targets and hence would be a good strategy against biofilms. Combinatorial approach against planktonic form of C. albicans has been studied and was found promising; however, only limited studies are available on biofilms.23 Our study is of significance as CQ combination with various antifungal drugs is studied in planktonic as well as biofilm growth forms. No significant change in MICs of antifungal drugs for planktonic growth of C. albicans was noted in presence of CQ, suggesting no synergistic activity against planktonic growth. Biofilm formation and mature biofilms were found resistant to the azoles, FLC and VOR (Table 1). While higher concentrations of CSP and AmB were required for preventive activity against biofilms than that of planktonic growth. Resistance to FLC and increased tolerance to VOR exhibited by early phase biofilms was totally reverted by the addition of CQ and biofilm formation became several folds more sensitive to these drugs. Similarly, mature biofilms which were resistant to azoles were found to be susceptible to these drugs in presence of CQ. Results of the study suggest that CQ can be developed as a promising partner molecule for combination therapy against biofilm. Although very effective with FLC and VOR, presence of CQ did not have significant synergism with CSP and AmB. No alteration in the sensitivity of developing as well as mature biofilms to CSP and AmB was evident in combination with CQ. Reasons behind synergistic activity only with two azoles need to be elucidated. Phase specific events are reported to be responsible for azole resistance in biofilms of C. albicans. Activation of drug efflux pumps in early phases and low membrane ergosterol content in mature phases of biofilms was found to play a major role in fluconazole resistance.6 Earlier workers have reported that CQ inhibits cholesterol synthesis in Wistar rats.24 In treatment of malaria, CQ binds to the haematin (a toxic by product of hemoglobin proteolysis containing iron as a center) and prevents its incorporation in to haemozoin. The free form of haematin may interfere with the normal membrane functions of parasite resulting in its inhibition.24 A study of Saccharomyces cerevisiae showed that CQ inhibited the yeast growth by iron deprivation.25 Similar effects of CQ in C. albicans may potentiate FLC and VOR against biofilm growth form. However, the exact mechanism remains to be elucidated. In summary, this in vitro study gives insight into use of CQ-azole combination against biofilms in C. albicans. The antifungal drugs FLC and VOR are commonly prescribed against candidiasis, as such reduction in their effective dosages after combination with CQ may be of wide interest. However, to confirm the utility of these drug combinations in clinics, in vivo studies are necessary.
Conflict of interestThe authors declare to have no conflict of interest.
RBS is thankful to ICMR, New Delhi, for fellowship provided to him under ICMR-SRF program (file no. 80/607/2008/ECD-I).