Sepsis is an illness with a high morbidity for which no effective treatment exists. Its treatment has a high cost because it usually requires an intensive care unit and expensive antibiotics. The present study focus in the production of reactive oxygen species in the early stages of sepsis. This study aimed at investigating the production of reactive oxygen specie during the inflammatory response in patients with sepsis.
MethodsReactive oxygen specie production and insoluble myeloperoxidase obtained from fresh whole blood were measured by photon counting chemiluminescence in the blood of 18 septic patients and 12 healthy individuals. Modified red blood cells were evaluated by staining of blood smears. The production of reactive oxygen species by macrophages and polymorphonuclear leukocytes put into contact with modified red blood cells were also assessed by photon counting chemiluminescence.
ResultsThe appearance of oxidatively modified erythrocytes, which is an evidence of oxidative stress, was supported by the detection of reactive oxygen species and insoluble myeloperoxidase in the whole blood of all septic patients. Peroxynitrite was the main reactive oxygen species found in the whole blood. Oxidatively modified erythrocytes activated phagocytic cells in vitro, leading to the considerable production of free radicals.
ConclusionIt was found that sepsis led to a high oxidative stress and to extensive modification of erythrocytes. It is proposed that a positive feedback mechanism, involving the activation of circulating leukocytes by these modified erythrocytes would maintain the pro-oxidative state even after the disappearance of bacteria.
Sepsis is widely recognized as a life-threatening organ dysfunction caused by dysregulated host response to infection.1 Sepsis is the leading cause of death in non-cardiac intensive care units around the world. Death rates are high, with 20% for sepsis, 40% for severe sepsis, and more than 60% for septic shock.2 Thus, it is essential to clarify the mechanisms that lead to lethality in this disease, so that they may become targets for therapies.
For most bacterial infections, polymorphonuclear neutrophils (PMNs) represent the first line of defense of the innate immune system. Phagocytosis of the microorganisms induces apoptosis in PMNs, which is dependent upon reactive oxygen species (ROS) production and is important for the resolution of infection and inflammation.3–5
Recently, studies of sepsis have expanded their focus to include microparticles formed as a result of PMN-derived insoluble myeloperoxidase (iMPO) endothelial and platelet cell activation. The resulting production of ROS, mainly hypochlorous acid generated by myeloperoxidase (MPO), impairs endothelial vascular function via increased oxidative stress and effects on the coagulation cascade.6,7
The present study sought to investigate the production of ROS during the inflammatory response in septic patients using chemiluminescence in real time.
Materials and methodsBlood donors and ethical considerationsThis study was approved by the Ethics Committee of the Complexo Hospitalar Universitário Professor Edgard Santos in Salvador, Brazil. During the period from March 2010 to December 2010, blood samples were collected from 12 healthy volunteers at the hospital and from 18 patients who were diagnosed with sepsis/septic shock and received antibiotic therapy in the intensive care unit. Informed consent was either sought from the patients, or, when not possible, from their next of kin.
Measurement of ROS in whole blood samplesROS levels in fresh whole blood samples were measured by chemiluminescence using a sensitive photon counter,8 with L-012 (Wako Pure Chemical Industries, Osaka, Japan) used as a secondary emitter. Whole blood samples without any prior separation were added to Petri dishes and diluted with Hanks’ balanced salt solution in a ratio of 1:1. After baseline reading for 200s, 50μM of L-012 was added to the plate and reading done after each sample reaches its plateau of photons emitted by L-012 activated by ROS for 100s. The effects of ROS were characterized using specific inhibitors, including 250μM of hydralazine and 1mM of desferrioxamine as inhibitors of peroxynitrite (ONOO−) and 9.4μM of superoxide dismutase (SOD) as inhibitor of superoxide and 40μM of sodium azide as inhibitor of MPO.
Detection of iMPO activityFor microparticle analysis, were collected 10mL of peripheral blood with heparin, donated by septic patients and healthy volunteers. The separation of plasma and erythrocytes was performed by centrifugation of whole blood at 290×g for 10min at 4°C. Then, the platelet-rich plasma (PRP) was centrifuged at 4500×g for 5min at 4°C. The supernatant was subjected to three subsequent ultracentrifugation 100,000×g at the TLA-100.3 rotor (Beckman Instruments Inc., Palo Alto, CA, USA) for 45min at 4°C. After ultracentrifugation the supernatant was discarded and the pellet containing the microparticles with MPO or iMPO was resuspended in Hank's balanced salt solution (HBSS).
MPO has strongly cationic loads and isoelectric point >10,9 and due to this characteristic, it is believed that MPO would be able to directly associate the microparticles by electrostatic interactions.10,11
MPO activity was assessed by estimating HOCl production using a previously described chemiluminescence method.12 Samples (resuspended pellet containing MPO) with known amounts of protein were placed in dishes, sealed with cling film and maintained at 37°C in a thermostatic light-sealed chamber. Their chemiluminescence emissions were then collected by reflections off a concave mirror and focused onto the photomultiplier tube. HOCl production derived for myeloperoxidase-hydrogen peroxidechloride system (MPO/H2O2/Cl− system) was recorded by luminescence after the addition of 50μM L-012. The HOCl and L-012 interaction derived chemiluminescence was measured in intact MPs using HBSS plus 10mM HEPES (pH 7.3) and 20μM H2O2. To confirm the luminescence dependency upon chloride, samples of MPs and L-012 were also prepared with HBSS without chlorine. Previously to analyses, baseline readings were performed, and chemiluminescence emission did not exceed 17counts/s.
For the collected blood smears, stains were prepared using Wright's stain and photographed using optical microscopy. From the same sample, erythrocytes were obtained by centrifugation at 290×g, fixed with half-strength Karnovsky's fixative and examined using a JEOL JSM 6390LV SEM (JEOL Ltd, Tokyo, Japan) electron microscope. The production of ROS by neutrophils and macrophages was assessed when the cells were challenged with freshly washed erythrocytes from patients and controls.
Visualization of erythrocytes derived from patients and healthy individualsErythrocytes were obtained from 2mL of blood (containing heparin) from septic patients or healthy volunteers. During collection, a drop of native blood was used for preparation of smears (stained with Wright's stain) and visualization of red blood cells by light microscopy. The blood samples were centrifuged at 290×g for separation of plasma and erythrocytes. The plasma was discarded, and the erythrocytes were washed by centrifugation under the same conditions.
Samples containing 106erythrocytes/mL were left still for 30min, followed by fixation in Karnovsky's fixative (2.5% glutaraldehyde and 4% paraformaldehyde in sodium cacodylate buffer 0.1M, pH 7.4) dehydrated in alcohol and stored on a glass surface for 16h at 4°C to be prepared for observation under a scanning microscope (JSM 6390 LV Low Vacuum; JEOL, Peabody, MA, USA). Images were obtained by secondary electron analysis at a working distance of 15–25mm and an accelerating voltage of 20–25kV.13
In vitro oxidation of erythrocytesErythrocytes were obtained as described above and washed twice by centrifugation under the same conditions. We evaluated if an in vitro model represented by the exposure of erythrocytes to ROS could mimic the in vivo situation of sepsis. To induce controlled oxidative damage to erythrocytes under experimental conditions, we chose well-studied and representative oxidizing agents: hydrogen peroxide (H2O2)+ Diamide. H2O2 is a water-soluble oxidant readily permeable to cell membranes and thought to primarily affect cytoplasmic components.14 Diamide can function as a thiol-oxidizing reagent and has been a popular candidate to stimulate oxidative stress in several other investigations.15
In the same concentration, 100μM of diamide and 100μM of H2O2 were added at 1.45×106cells/mL to suspension of erythrocytes. Untreated erythrocytes were considered negative control. An aliquot was taken from each sample for smear preparation and visualization of the oxidatively modified erythrocytes by optical microscopy. Part of the samples were left still for 30min at 37°C and then fixed with Karnovsky's fixative (2.5% glutaraldehyde and 4% paraformaldehyde in sodium cacodylate buffer 0.1M, pH 7.4) dehydrated in alcohol and stored on a glass surface for 16h at 4°C to be prepared for observation under a scanning electron microscopy (JSM 6390 LV Low Vacuum; JEOL, Peabody, MA, USA) as described elsewhere.13
Production of ROS by J774 macrophages and neutrophils stimulated by oxidized erythrocytesErythrocytes were obtained from peripheral blood of septic patients as described above and diluted at 1.45×106erythrocytes/mL with HBSS at a ratio of 1:5. The positive control was performed at 1.45×106erythrocytes/mL of healthy volunteers, which were diluted and treated with a strong oxidizer, nitrite peroxide (ozone reaction, ∼5% oxygen with sodium azide in water 0.02–0.2M, pH12) every 5min three times for 1h,16 and the untreated erythrocytes were considered as negative control.
Each coverslip with attached cells was transferred to a Petri dish with addition of RPMI complete culture medium and left at 37°C for 1h. An aliquot of the erythrocyte suspension of each sample was taken for analysis of ROS production during phagocytosis and detected by chemiluminescence in the presence of L-012. To test the cellular response to ROS production, the J774 macrophages were stimulated with phorbol 12-myristate 13-acetato (PMA) and measure of ROS production by chemiluminescence dependent on L-012.
Measure of ROS produced by neutrophils in erythrocyte presenceErythrocytes were obtained from heparinized blood of healthy volunteers and septic patients. Blood samples were subjected to centrifugation to separate the plasma and erythrocytes. The erythrocytes were washed twice by centrifugation under the same conditions. 1.45×106erythrocytes/mL in septic patients were diluted and left for 1h. The positive controls were treated in the following ways: erythrocytes from healthy volunteers were treated with calcium ionophore and nitrite peroxide. Untreated cells were considered as negative control. After standing 1h at 37°C, the samples were centrifuged and the pellet was resuspended. 1.25×107neutrophils/mL obtained from whole blood of healthy volunteers were placed on treated and untreated erythrocytes in a ratio of 1:5 and left at 37°C for 10min. An aliquot of 100mL of the mixture of each sample was diluted with in Petri dish to detect the production of ROS by neutrophils during phagocytosis by chemiluminescence dependent on L-012 (50μM). The remaining aliquot of each sample remained at 37°C for 1h, followed by fixation with Karnovsky's fixative for observation by scanning electron microscopy.
Visualization of erythrocytes phagocytosis by macrophages and neutrophilsAfter measurement of ROS by chemiluminescence as detailed above, the red cell and J774 macrophage mixtures or red cell and neutrophil mixtures were incubated at 37°C for 1h. They were then fixed with Karnovsky's fixative for 16h at 4°C and prepared for observation by scanning electron microscopy as described elsewhere.
Statistical analysisThe data distribution was tested for Gaussian distribution by the D’Agostino's and Pearson's test. Variables of the two experimental groups were compared using the Mann–Whitney test. For more than three experimental groups the Kruskal–Wallis test was used, followed by Dunn's test. Differences were considered statistically significant when p-value≤0.05.
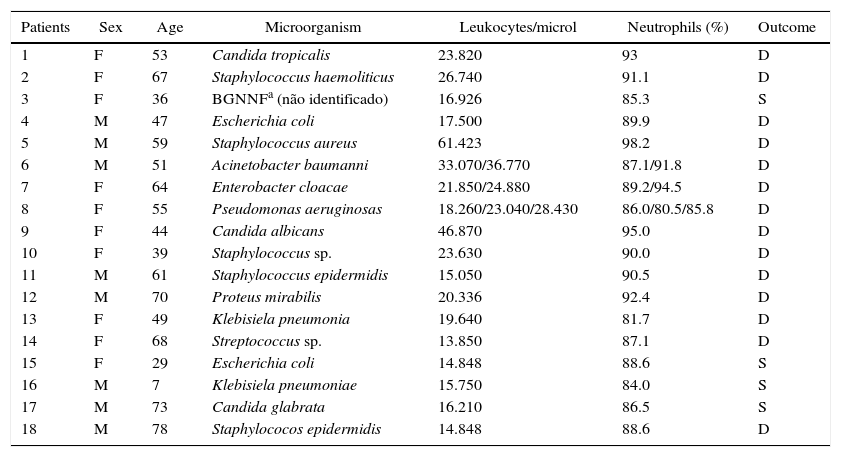
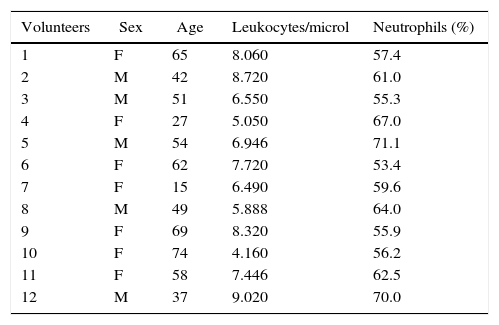
ResultsQuantitative hematological abnormalities in septic patientsEighteen septic patients (10 women and 8 men) aged 7–75 years (52.7±17.6) and 12 healthy volunteers (7 women and 5 men) aged 15–74 years (50.2±17.5) participated in the study (Tables 1 and 2). With regard to the hematological parameters, there was significantly more leukocytosis and neutrophilia in the septic patients (24±12leukocytes/μL, 89.5±4% neutrophils) compared to the healthy volunteers (7±1.5leukocytes/μL, 61±6% neutrophils) (p<0.001). In addition, the septic patients had lower values of hemoglobin, hematocrit, number of erythrocytes, and platelets in relation to the controls (data not shown).
Clinical data of septic patients in the study.
| Patients | Sex | Age | Microorganism | Leukocytes/microl | Neutrophils (%) | Outcome |
|---|---|---|---|---|---|---|
| 1 | F | 53 | Candida tropicalis | 23.820 | 93 | D |
| 2 | F | 67 | Staphylococcus haemoliticus | 26.740 | 91.1 | D |
| 3 | F | 36 | BGNNFa (não identificado) | 16.926 | 85.3 | S |
| 4 | M | 47 | Escherichia coli | 17.500 | 89.9 | D |
| 5 | M | 59 | Staphylococcus aureus | 61.423 | 98.2 | D |
| 6 | M | 51 | Acinetobacter baumanni | 33.070/36.770 | 87.1/91.8 | D |
| 7 | F | 64 | Enterobacter cloacae | 21.850/24.880 | 89.2/94.5 | D |
| 8 | F | 55 | Pseudomonas aeruginosas | 18.260/23.040/28.430 | 86.0/80.5/85.8 | D |
| 9 | F | 44 | Candida albicans | 46.870 | 95.0 | D |
| 10 | F | 39 | Staphylococcus sp. | 23.630 | 90.0 | D |
| 11 | M | 61 | Staphylococcus epidermidis | 15.050 | 90.5 | D |
| 12 | M | 70 | Proteus mirabilis | 20.336 | 92.4 | D |
| 13 | F | 49 | Klebisiela pneumonia | 19.640 | 81.7 | D |
| 14 | F | 68 | Streptococcus sp. | 13.850 | 87.1 | D |
| 15 | F | 29 | Escherichia coli | 14.848 | 88.6 | S |
| 16 | M | 7 | Klebisiela pneumoniae | 15.750 | 84.0 | S |
| 17 | M | 73 | Candida glabrata | 16.210 | 86.5 | S |
| 18 | M | 78 | Staphylococos epidermidis | 14.848 | 88.6 | D |
M, male; F, female; D, death; S, survival.
Clinical data of healthy subjects included in the study.
| Volunteers | Sex | Age | Leukocytes/microl | Neutrophils (%) |
|---|---|---|---|---|
| 1 | F | 65 | 8.060 | 57.4 |
| 2 | M | 42 | 8.720 | 61.0 |
| 3 | M | 51 | 6.550 | 55.3 |
| 4 | F | 27 | 5.050 | 67.0 |
| 5 | M | 54 | 6.946 | 71.1 |
| 6 | F | 62 | 7.720 | 53.4 |
| 7 | F | 15 | 6.490 | 59.6 |
| 8 | M | 49 | 5.888 | 64.0 |
| 9 | F | 69 | 8.320 | 55.9 |
| 10 | F | 74 | 4.160 | 56.2 |
| 11 | F | 58 | 7.446 | 62.5 |
| 12 | M | 37 | 9.020 | 70.0 |
M, male; F, female.
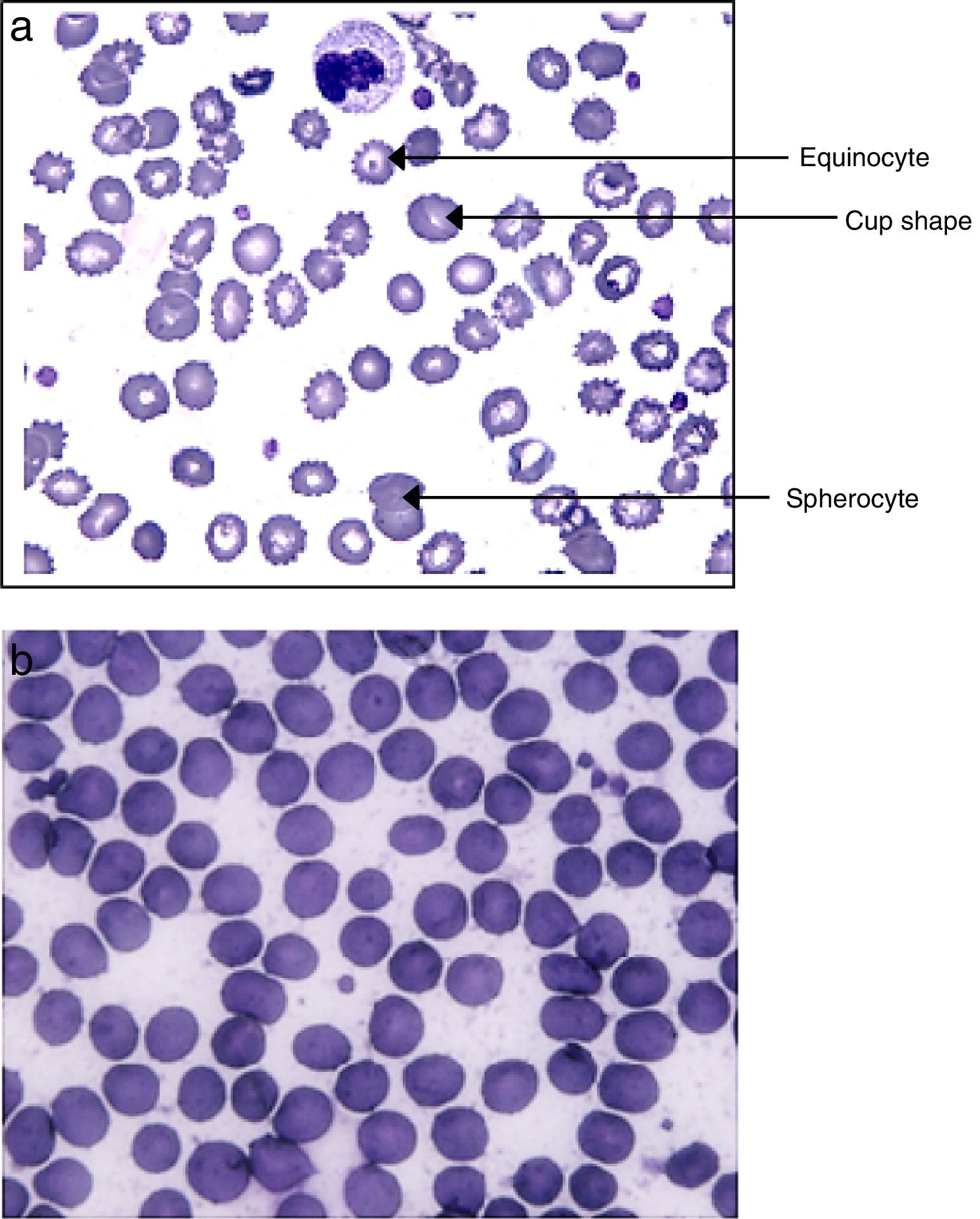
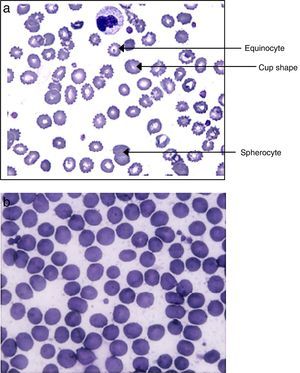
As shown in Fig. 1A, modified erythrocytes, such as spherocytes, cup-shaped cells and echinocytes were ubiquitous in the blood of septic patients but absent in volunteers (Fig. 1B).
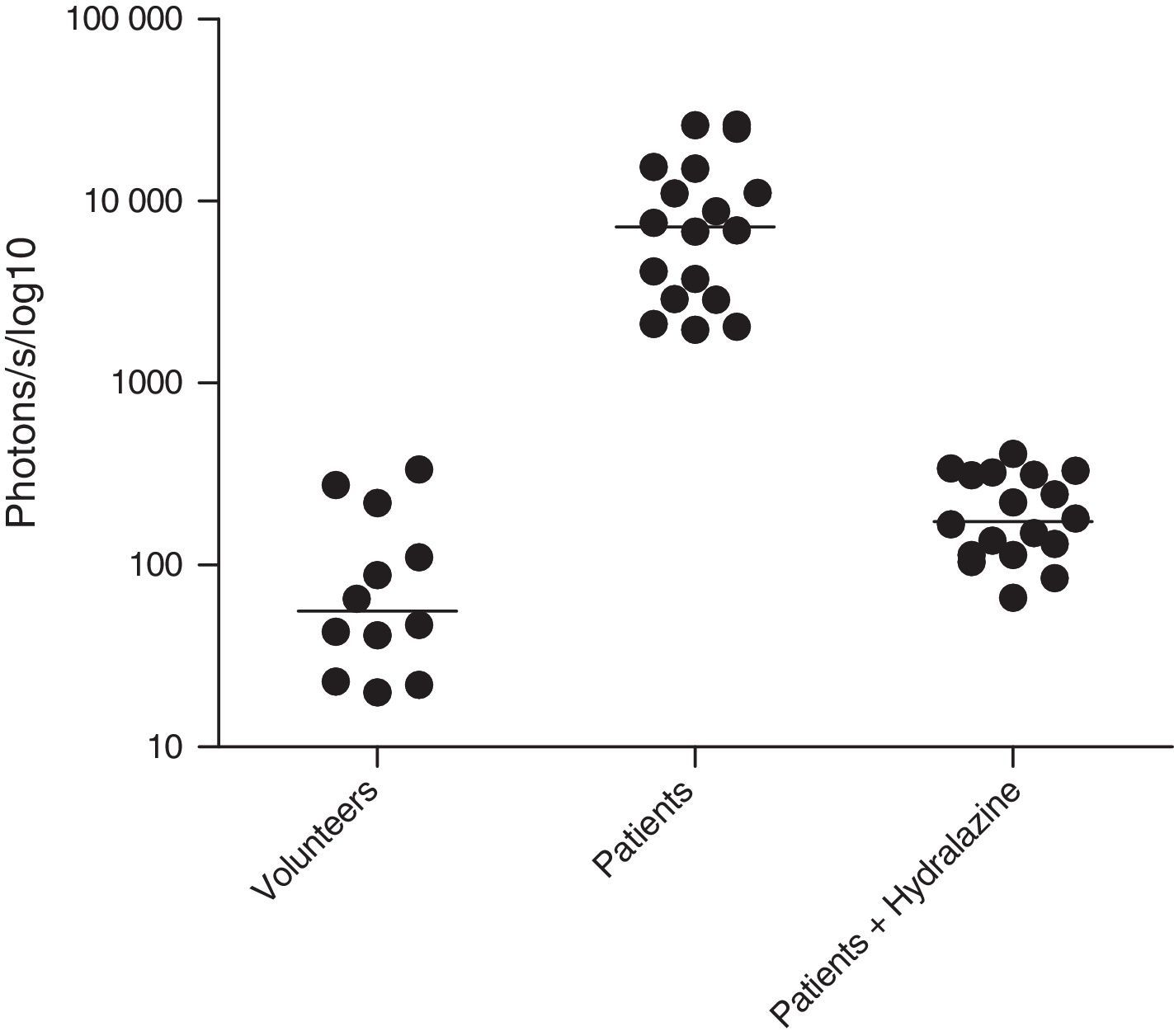
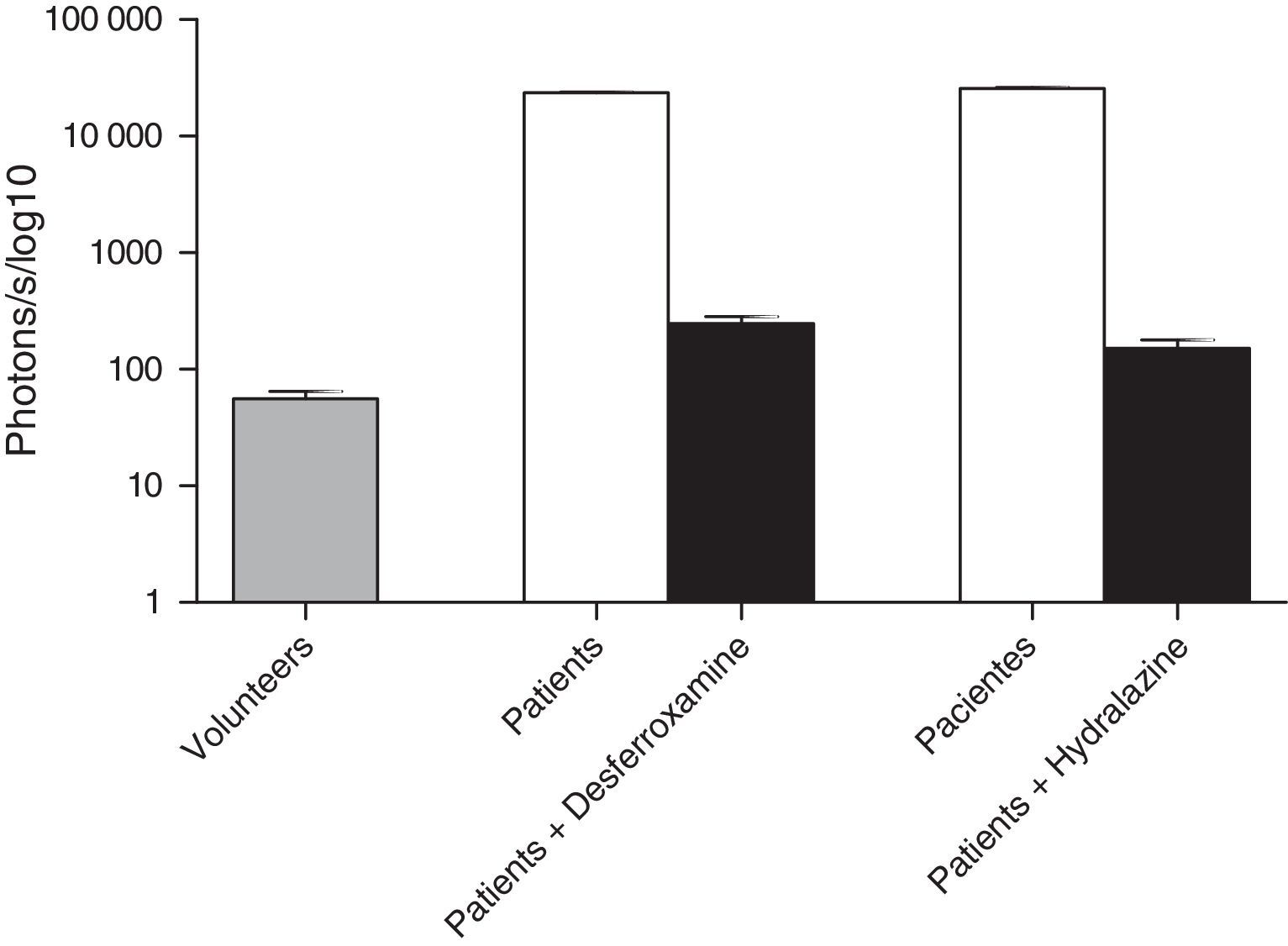
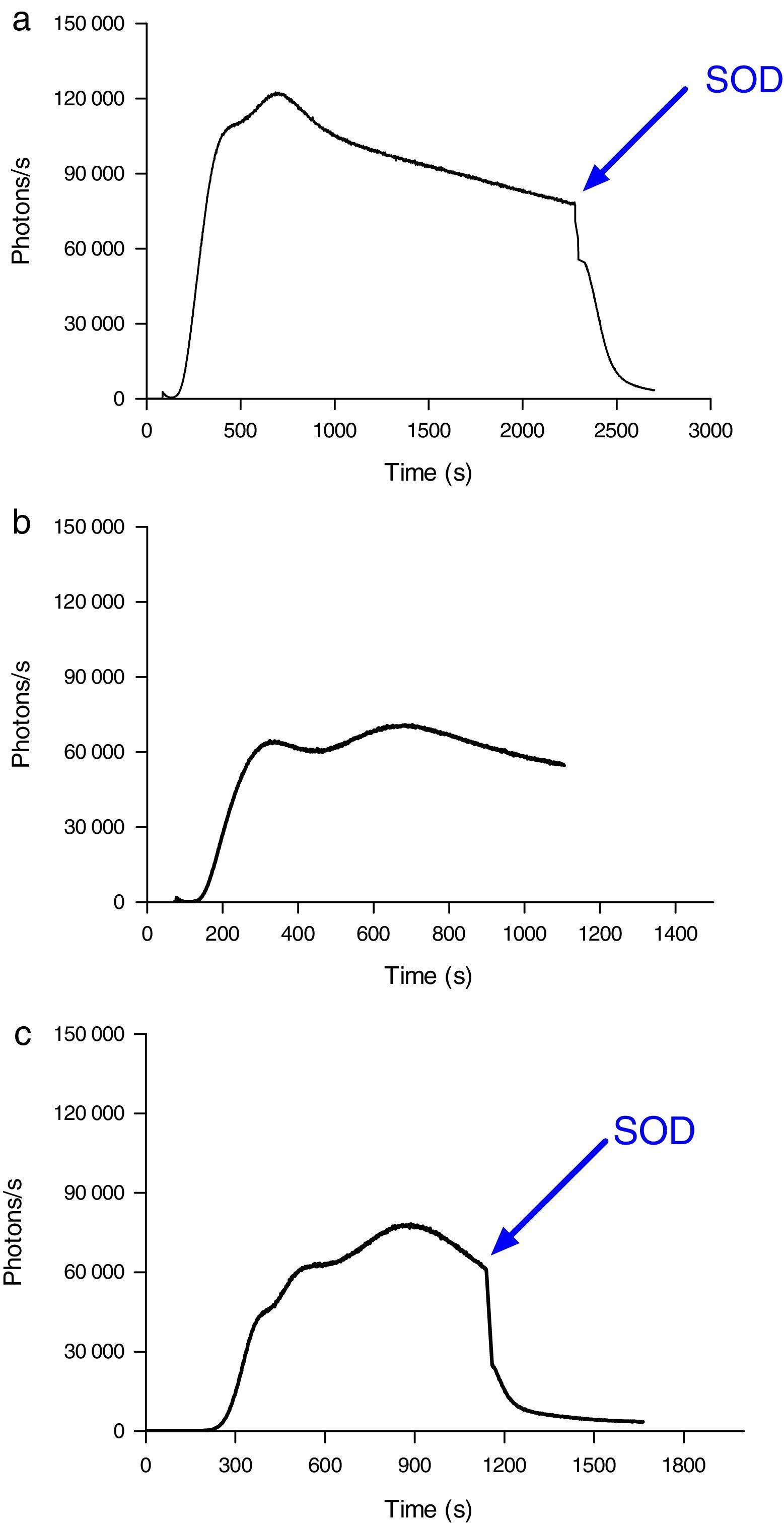
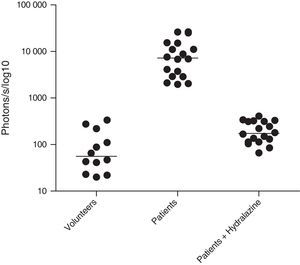
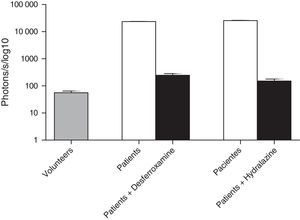
ROS levels in septic patientsThe 18 septic patients had higher ROS levels in the blood than the 12 healthy volunteers (Fig. 2). Furthermore, ONOO− was shown to be the main ROS component. This was demonstrated by showing that desferrioxamine and hydralazine inhibited the oxidative reaction (Fig. 3), as both are scavengers for ONOO−.17,18
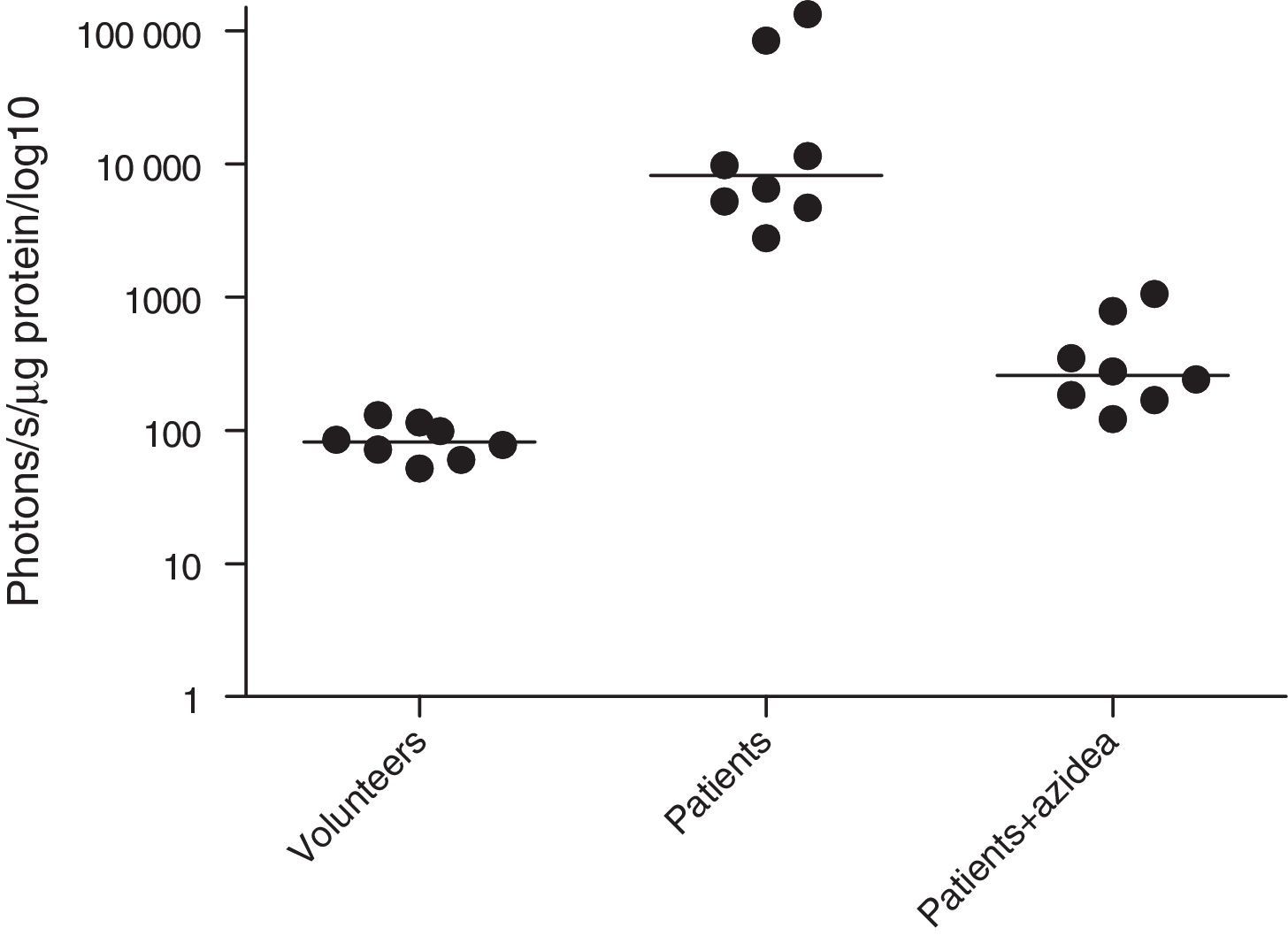
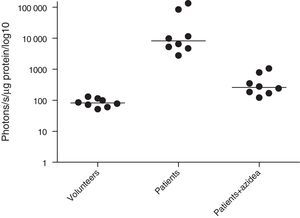
MPO activity was measured in microparticles obtained from the plasma of septic patients. The levels of iMPO activity in those patients were significantly higher than the levels observed in healthy volunteers. The addition of sodium azide to this assay resulted in a significant decrease in photon detection, indicating that the MPO was functionally active (Fig. 4).
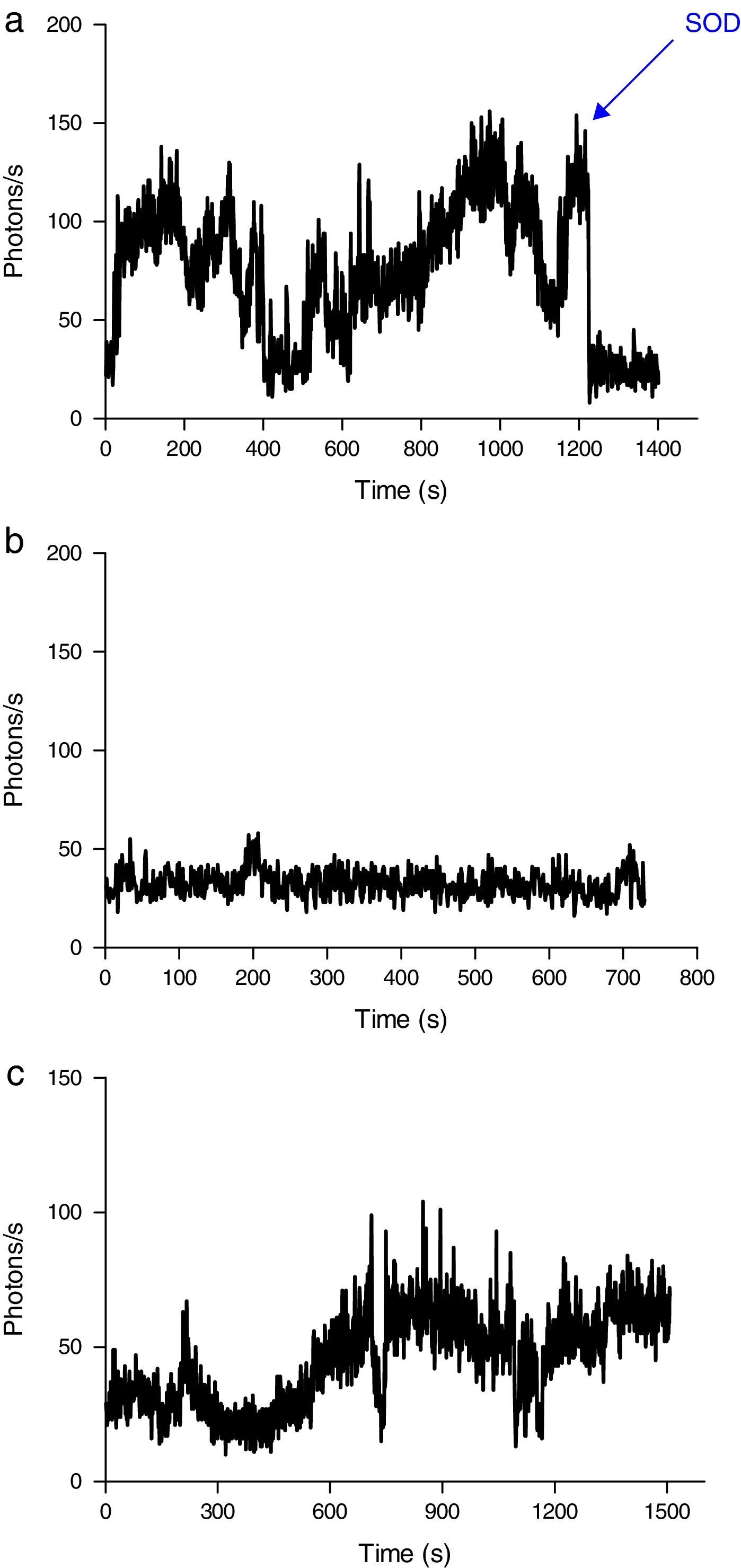
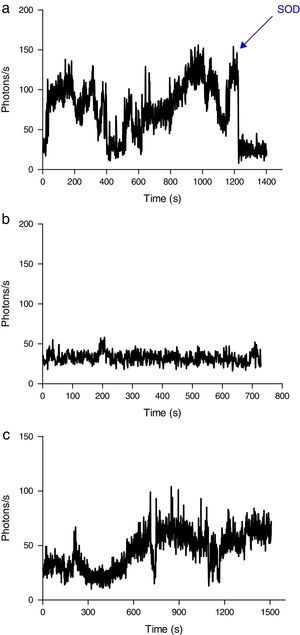
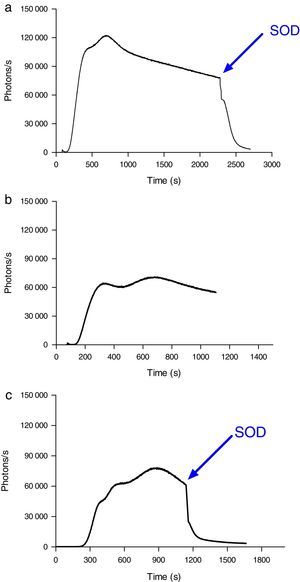
Induction of ROS production by phagocytes by incubation with oxidized erythrocytesWhen erythrocytes from septic patients were added to cultured J774 macrophages (Fig. 5A) and neutrophils (Fig. 6A), an increase in ROS production was observed. Addition of SOD to the culture resulted in a reduction in the emission of light. J774 macrophages placed in contact with erythrocytes from healthy volunteers led to a ROS production (photons/s) that remained at a basal level (Fig. 5B). For neutrophils cultured under the same conditions, the emitted light was reduced by two-thirds (Fig. 6B) compared with the ROS production in the presence of erythrocytes from septic patients. Moreover, using oxidatively modified erythrocytes in vitro, the pattern of ROS production with J774 macrophages and neutrophils was similar (Figs. 5C and 6C).
ROS production by J774 macrophages during contact with erythrocytes, as measured by chemiluminescence. (A) ROS production by macrophages in the presence of erythrocytes from septic patients. The SOD inhibitor of superoxido was added to some samples. (B) Basal photon counts/second observed when J774 macrophages were put into contact with erythrocytes of healthy volunteers. (C) ROS production by macrophages during contact with erythrocytes that had been oxidatively modified in vitro by treatment with peroxynitrite. The results are representative of three patients.
ROS production by neutrophils during contact with erythrocytes, as measured by chemiluminescence. (A) ROS production by neutrophils in the presence of erythrocytes from septic patients. The SOD inhibitor of superoxido was added to some samples. (B) ROS production by neutrophils in the presence of erythrocytes from healthy volunteers. (C) ROS production by neutrophils during contact with erythrocytes that had been oxidatively modified in vitro by treatment with peroxynitrite. The SOD inhibitor of superoxido was added to some samples. The results are representative of three patients.
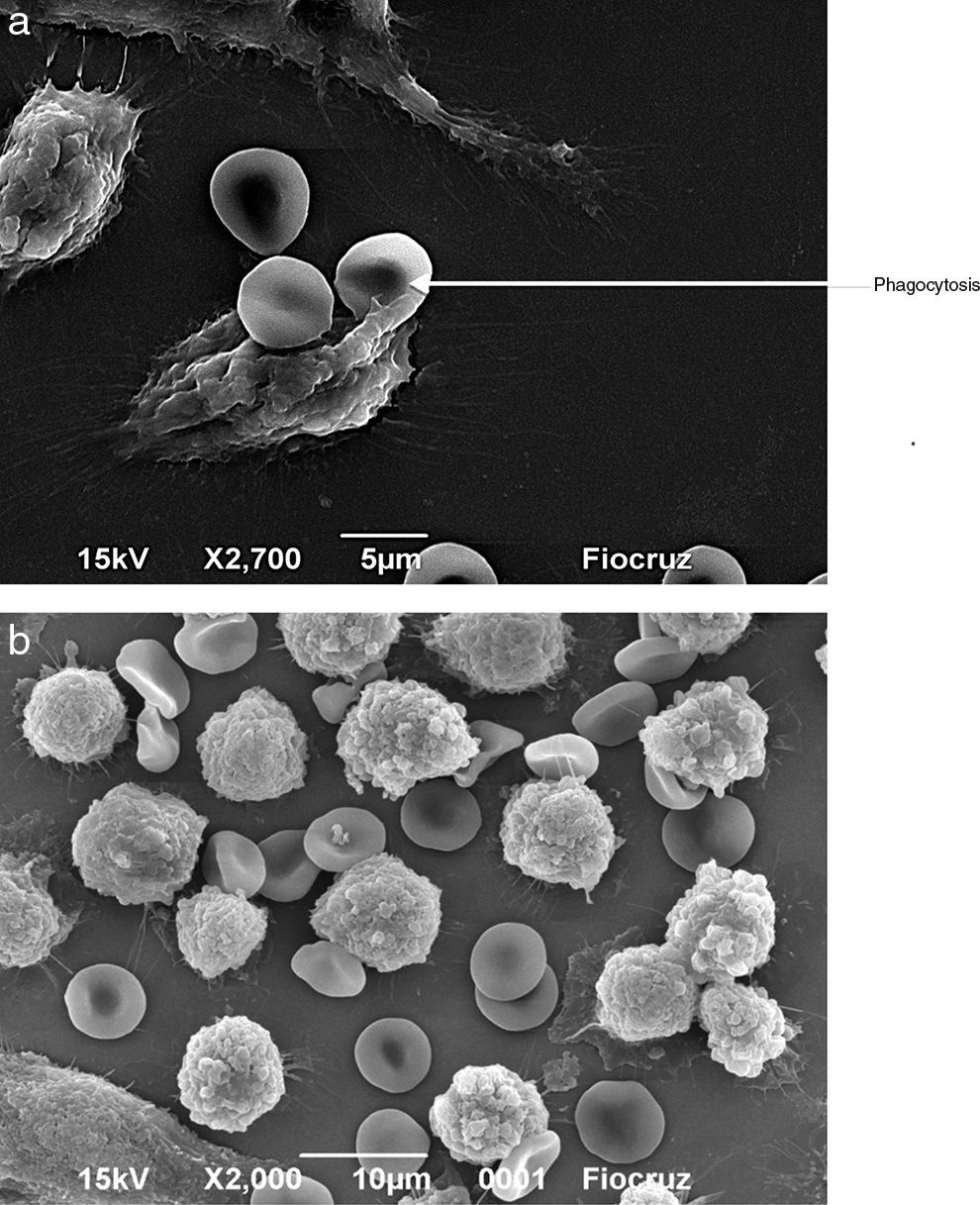
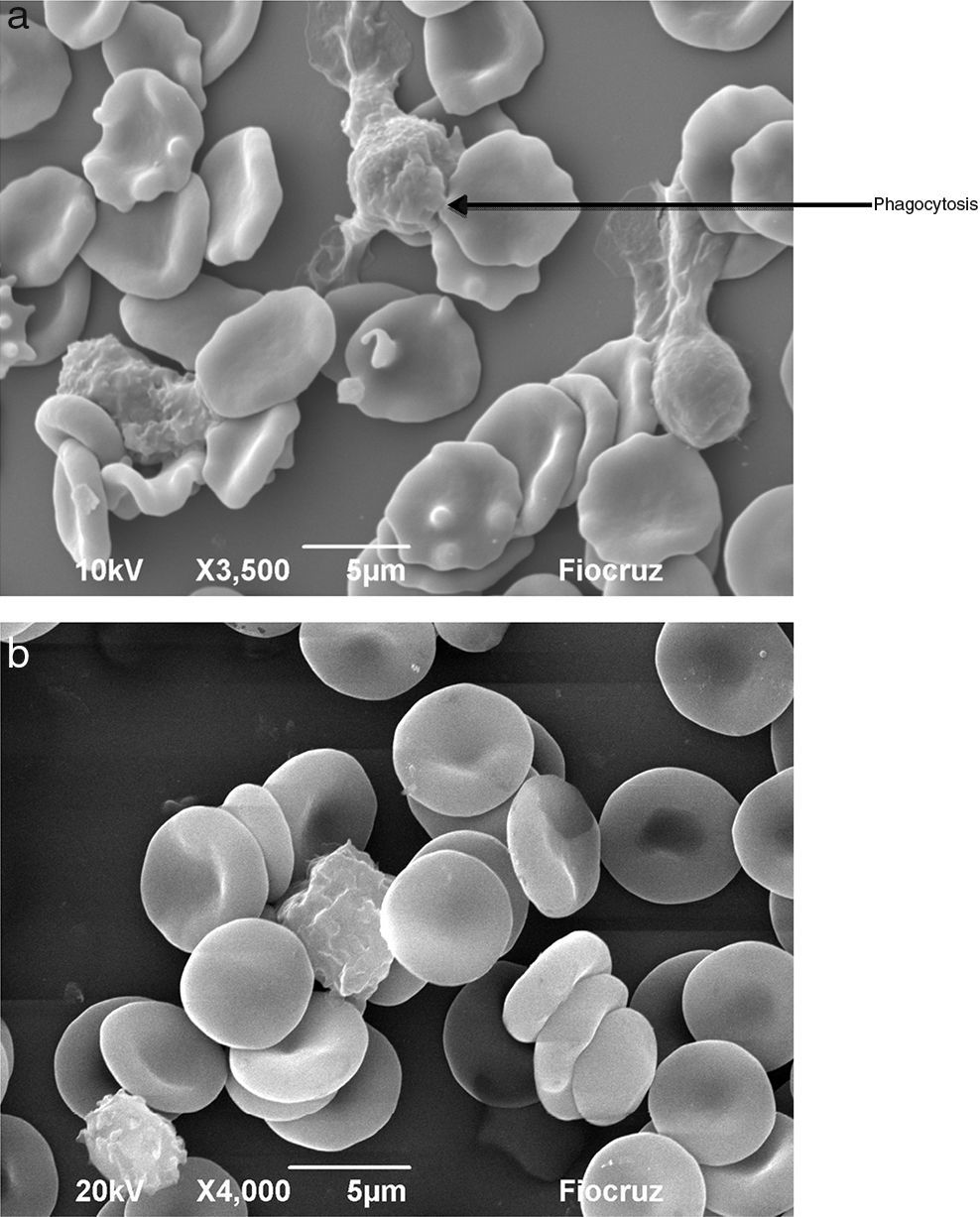
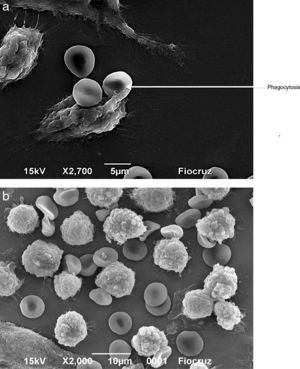
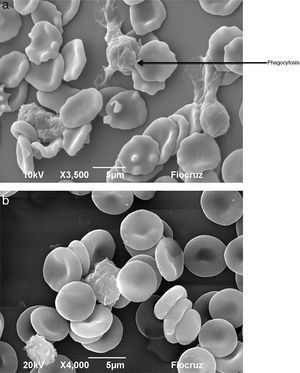
Macrophages (Fig. 7A) and neutrophils (Fig. 8A) phagocytized erythrocytes obtained from septic patients; in contrast, red blood cells (RBCs) from healthy volunteers were not phagocytized (Figs. 7B and 8B).
Visualization by scanning electron microscopy of the phagocytosis of erythrocytes by J774 macrophages. Macrophages and erythrocytes were incubated for 1h at 37°C and then fixed with Karnovsky. (A) Adhesion and phagocytosis of oxidatively modified erythrocytes from a septic patient by J774 macrophages. (B) Erythrocytes from healthy volunteer in presence of J774 macrophages.
Visualization by scanning electron microscopy of phagocytosis of erythrocytes by neutrophils. The cells were incubated for 1h at 37°C and then fixed with Karnovsky. (A) Phagocytosis of oxidatively modified erythrocytes by neutrophils from a septic patient. (B) Erythrocytes from healthy volunteer in presence of neutrophils.
The high mortality rate of sepsis patients (77.7% of deaths in the present study, for instance) shows that the current treatments are ineffective and highlights a gap in the knowledge of the triggers of the inflammatory process. In this work, we describe the production of ROS during sepsis. This should be ascribed to the continual phagocytosis and degradation of the pathogen by PMNs or by activation of these cells with pathogen’ products.3,19
The levels of ROS could be reduced by the presence of the inhibitors hydralazine and desferrioxamine, which act by specifically inhibiting the radical peroxynitrite showing that peroxynitrite may play a role in the oxidative stress that occurs in sepsis.20,21
We sought to investigate the presence of microparticles in the plasma of septic patients. These microparticles are a product of cell activation, primarily of monocytes and neutrophils, that occurs during the inflammatory process.22 They contain the MPO enzyme, which produces several powerful oxidants and protein modifiers in the presence of its substrate, the hydrogen peroxide. This enzyme is recognized as the major antibacterial factor in circulating inflammatory cells, especially neutrophils.23 This preliminary study clearly demonstrates an association between iMPO and sepsis, although it was not possible to establish a direct correlation between elevated iMPO and the severity of sepsis. The oxidants (ROS) that are produced by these microparticles are likely involved in both the oxidation of RBCs and the damage to vessel walls.
Activated PMNs may go beyond an initial bactericidal effect and contribute to other adverse effects, such as cell and tissue alteration, during a prolonged period of uncontrolled inflammation.
In sepsis, inflammation resulting from ROS production promotes morphological changes in erythrocytes, with the appearance of spherocytes, cup-shaped cells, and especially echinocytes. These oxidatively modified erythrocytes were observed in blood samples obtained from septic patients using optical microscopy and scanning electron microscopy. These morphological changes have been shown to be the result of lipid peroxidation and changes to integrin and cytoplasmic proteins, mainly aminophospholipids, leading to alterations of the cell membrane.24
Modified erythrocytes have also been observed in patients with diagnosis of Alzheimer's disease,25 sickle cell anemia,26 chronic obstructive pulmonary disease,27 chronic kidney disease.28 As shown in the present work, these oxidatively modified erythrocytes proved to be a powerful signal for ROS production by macrophages and neutrophils in vitro.
One unanswered question in relation to sepsis is how this condition remains even after blood-derived bacteria have been eliminated by therapeutic intervention. Based on our results, we suggest that the production of ROS in the blood of septic patients is aggravated by the interaction of phagocytes with modified red blood cells (mRBCs), which would originate from the excessive production of ROS as a consequence of the initial contact of bacteria and their products with PMNs. These mRBCs would induce the continuous activation of PMNs and other phagocytic cells (Figs. 5C and 6C), with increased production of ROS, which would generate a positive feedback mechanism. This mechanism may lead to the typical pathology of sepsis/septic shock, the expression of NADPH oxidase in the endothelium, the deformity of erythrocytes, increased hematocrit, microcirculation failure, and organ damage.29 However, the proposed feedback for inflammation amplification is only inferred and more studies should be conducted to clarify these questions.
In conclusion, the present results, may further our understanding of the high mortality rate in sepsis. It was found that sepsis led to a high oxidative stress and to extensive modification of erythrocytes. Our findings provided evidence to suggest the production of ROS during inflammatory response in septic patients as well as morphological alterations of erythrocytes during the inflammatory process, and that this modified cells are phagocytized by macrophages and PMNs. However, causal relation that mRBCs could aggravate the septic process must be further investigated.
Specific inhibitors of MPO and/or ONOO− quenchers should be investigated for the treatment of sepsis. Natural and renewable antioxidants, such as glutathione, which connects to the ethyl radical and thereby permeates the cell membrane could be taken into consideration. However, further studies should be carried out, especially those concerning the possible pathogenic role of nitric oxide.
Conflicts of interestThe authors declare no conflicts of interest.
The authors would like to thank FIOCRUZ-Bahia (Centro de Pesquisas Gonçalo Moniz) for allowing the experiments which needed to be done, FAPESB for the financial support as well as the Institutions and individuals who agreed to take part in this Project.