This study aims to investigate the antimicrobial and the anti-biofilm activities of Lactobacillus plantarum extract (LPE) against a panel of oral Staphylococcus aureus (n=9) and S. aureus ATCC 25923. The in vitro ability of LPE to modulate bacterial resistance to tetracycline, benzalchonium chloride, and chlorhexidine were tested also.
MethodsThe minimum inhibitory concentrations (MICs) and the minimal bactericidal concentrations of Lactobacillus plantarum extract, tetracycline, benzalchonium chloride and clohrhexidine were determined in absence and in presence of a sub-MIC doses of LPE (1/2 MIC). In addition, the LPE potential to inhibit biofilm formation was assessed by microtiter plate and atomic force microscopy assays. Statistical analysis was performed on SPSS v. 17.0 software using Friedman test and Wilcoxon signed ranks test. These tests were used to assess inter-group difference (p<0.05).
ResultsOur results revealed that LPE exhibited a significant antimicrobial and anti-biofilm activities against the tested strains. A synergistic effect of LPEs and drug susceptibility was observed with a 2–8-fold reduction.
ConclusionLPE may be considered to have resistance-modifying activity. A more detailed investigation is necessary to determine the active compound responsible for therapeutic and disinfectant modulation.
Staphylococci are important causes of infections associated with various devices. Staphylococcus aureus is one of the most frequent human pathogen associated to medical implant.1 It has been isolated from parotitis,2 gingival pockets,3 periodontitis,4 carious lesions,5 and gingivitis.6 Orthodontic and other oral appliances may act as reservoir of resistant opportunistic S. aureus.7
S. aureus has the capacity to adhere to various medical devices and form biofilm.8 Biofilm is associated to the ica gene encoding for polysaccharide intercellular adhesion (PIA)9 which leads to increased bacterial drug resistance compared to planktonic cells.10,11
S. aureus is able to acquire resistance to antibiotics and to antiseptic agents via efflux pumps (TetK, msrA transporters) systems, which export certain tetracyclines and macrolides molecules outside the cells. On the other hand, some multidrug resistance (MDR) proteins like NorA and QacA confer resistance to a wide range of structurally unrelated antiseptics.12 In clinical practice, chlorhexidine (CHX) and quaternary ammonium compounds (QAC) are the most frequently used disinfectants to reduce and prevent the spread of pathogens. Multidrug resistance pumps have been recognized as mediators of a number of commonly used ammonium compounds and detergents.13 However, the use of these disinfectants in hospitals may contribute to the rise of disinfectant-resistant bacteria14,15 due to QAC-resistant genes (qacA, qacB, and qacC), which have been identified in several staphylococcal species.16–18
In this study, the antibacterial activity of Lactobacillus plantarum extract (LPE) was investigated. In addition, the ability of LPE to modulate the susceptibility of S. aureus, isolated from the oral cavity of Tunisian children, to tetracycline (TET), benzalchonium chloride (BC) and clohrhexidine (CHX) was tested. Secondly, the effects of disinfectant and antibiotic associated with LPE were tested for biofilm inhibition of S. aureus to polystyrene and glass using microtiter plate and atomic force microscopy assays, respectively.
Material and methodsAntibacterial activity of L. plantarumL. plantarum strain was isolated from Tunisian traditional fermented milk (ricotta cheese), identified with a conventional method using Api-50 CHL system (BioMerieux, Marcy-l’Étoile, France) and by polymerase chain reaction (PCR) technique using L. plantarum-specific primers: forward IDL04F: 5′-AGGGTGAAGTCGTAACAAGTAGCC-3′ and reverse IDL62R 5′-CTAGTGGTAACAGTTGATTAAAACTGC-3′, giving a product size of about 428bp as described previously.19
Cell-free supernatant was obtained by centrifuging (10,000×g for 10min at 4°C) L. plantarum culture, grown in MRS broth for 16h at 30° C. The supernatant was adjusted at neutral pH and filter-sterilized (0.22μm, Millipore). The obtained L. plantarum extract (LPE) was conserved at 4°C until use.
Antibacterial activity of LPE was tested against nine strains (Table 1) isolated from the oral cavity of Tunisian children and S. aureus ATCC 25923 using the broth microdilution method.20,21 The LPE was tested alone or in combination with antibiotics.
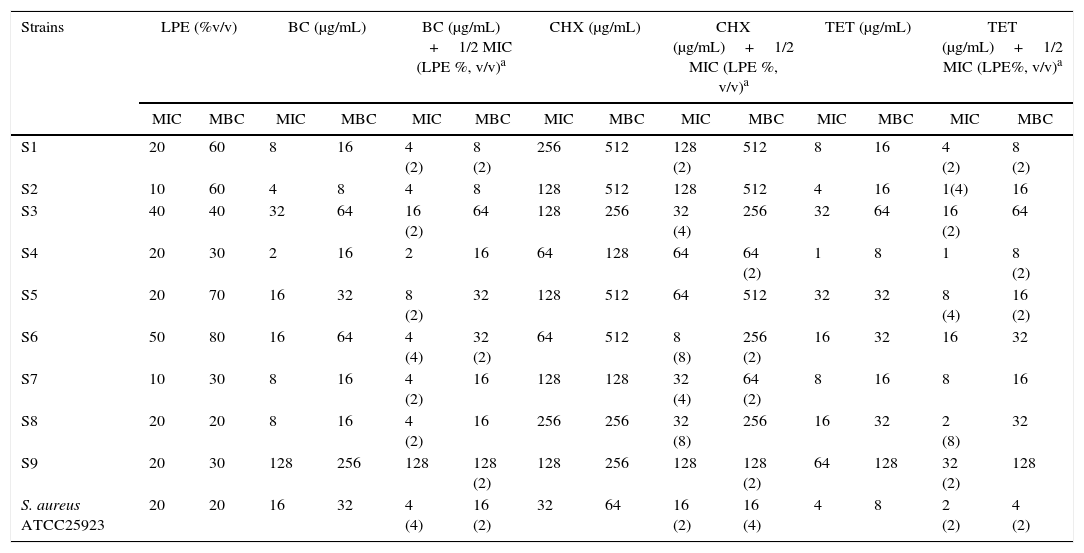
Minimum inhibitory and minimum bactericidal concentrations in μg/mL of Lactobacillus plantarum extract alone or in combination with benzalchonium chloride, chlorhexidine, and tetracycline.
| Strains | LPE (%v/v) | BC (μg/mL) | BC (μg/mL) +1/2 MIC (LPE %, v/v)a | CHX (μg/mL) | CHX (μg/mL)+1/2 MIC (LPE %, v/v)a | TET (μg/mL) | TET (μg/mL)+1/2 MIC (LPE%, v/v)a | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| MIC | MBC | MIC | MBC | MIC | MBC | MIC | MBC | MIC | MBC | MIC | MBC | MIC | MBC | |
| S1 | 20 | 60 | 8 | 16 | 4 (2) | 8 (2) | 256 | 512 | 128 (2) | 512 | 8 | 16 | 4 (2) | 8 (2) |
| S2 | 10 | 60 | 4 | 8 | 4 | 8 | 128 | 512 | 128 | 512 | 4 | 16 | 1(4) | 16 |
| S3 | 40 | 40 | 32 | 64 | 16 (2) | 64 | 128 | 256 | 32 (4) | 256 | 32 | 64 | 16 (2) | 64 |
| S4 | 20 | 30 | 2 | 16 | 2 | 16 | 64 | 128 | 64 | 64 (2) | 1 | 8 | 1 | 8 (2) |
| S5 | 20 | 70 | 16 | 32 | 8 (2) | 32 | 128 | 512 | 64 | 512 | 32 | 32 | 8 (4) | 16 (2) |
| S6 | 50 | 80 | 16 | 64 | 4 (4) | 32 (2) | 64 | 512 | 8 (8) | 256 (2) | 16 | 32 | 16 | 32 |
| S7 | 10 | 30 | 8 | 16 | 4 (2) | 16 | 128 | 128 | 32 (4) | 64 (2) | 8 | 16 | 8 | 16 |
| S8 | 20 | 20 | 8 | 16 | 4 (2) | 16 | 256 | 256 | 32 (8) | 256 | 16 | 32 | 2 (8) | 32 |
| S9 | 20 | 30 | 128 | 256 | 128 | 128 (2) | 128 | 256 | 128 | 128 (2) | 64 | 128 | 32 (2) | 128 |
| S. aureus ATCC25923 | 20 | 20 | 16 | 32 | 4 (4) | 16 (2) | 32 | 64 | 16 (2) | 16 (4) | 4 | 8 | 2 (2) | 4 (2) |
LPE, Lactobacillus plantarum extract; BC, benzalchonium chloride; CHX, chlorhexidine; TET, tetracycline; MIC, minimal inhibitory concentration; MBC, minimal bactericide concentration.
The S. aureus strains (n=9) used in this study were isolated from the oral cavity of Tunisian children from the dental clinic of dentistry (Monastir, Center of Tunisia).
The criteria for inclusion were: no antibiotic treatment four weeks prior to sampling, no use of mouth rinses or any other preventive measure that might involve exposure to antimicrobial agents, and no systemic disease. A sterile swab was used for sample collection from the oral cavity of each patient. After incubation (24h at 37°C), swabs were plated on blood agar plates supplemented with 5% sheep blood (24h at 37°C) and bacterial identification was achieved using conventional methods.
Minimal inhibitory concentration determinationThe broth microdilution method was used to determine the minimum inhibitory concentration (MIC) of LPE (5–90%, v/v), BC (Acros organics, USA) (0–1024μg/mL), CHX (Sigma–Aldrich, USA) (0–1024μg/mL) and tetracycline (TET) (0–1024μg/mL) according to the Clinical and Laboratory Standards Institute (2006) guidelines.22
After 24h of incubation, bacterial growth was evaluated by the presence of turbidity and a pellet on the well bottom. MIC was defined as the lowest concentration of the compound that had no macroscopically visible growth. All experiments were carried out three times.
Minimal bactericidal concentration determination (MBC)To determine the MBC values, 10μL of each well medium, with no visible growth was removed and inoculated in Muller Hinton agar plates. MBC was defined as the lowest concentration at which 99% of the bacteria were killed. Each experiment was repeated at least twice.23
Modulation of S. aureus susceptibility to BC, CHX and TET by LPETo determine the potential effect of LPE to modulate drug resistance of S. aureus, MICs of BC, CHX and TET (ranging from 0.5 to 2048μg/mL) were determined alone and combined with a sub-MIC of LPE (1/2 MIC, v/v) using the microtiter plates assay.24
Determination of anti-biofilms activity by microtiter plates assayThe anti-adhesion properties of LPE (ranging from 10% to 90%, v/v) to S. aureus strains were tested as previously described by Merritt et al.25 Briefly, the bacterial culture was grown in Tryptone soy broth (TSB) at 37°C for 24h. Two microliters were disposed into each well of 96-well plates in the presence of 198μL of the medium supplemented with 2% glucose (w/v) containing LPE. Plates were incubated in aerobic conditions at 37°C for 24h. The anti-biofilm activity of TET, CHX, and BC ranging from 2 to 1024μg/mL were determined against S. aureus strains with or without sub-MIC of the LPE (1/2 MIC, v/v), using the microtiter plates assay. After incubation, the plate was washed with phosphate buffer saline, stained with 100μL of 1% (w/v) crystal violet and incubated at room temperature for 15min. Then, the absorbance at 590nm was determined using a microplate reader (D.E.E.D reader, Bio-Rad instrument). The mean absorbance (OD590nm) of the sample was determined, and the percentage of inhibition obtained for each concentration of LPE and drugs was determined by the following formula:
Evaluation of LPE biofilm inhibition by atomic force microscopy (AFM)To evaluate biofilm inhibition potency of LPE, S. aureus ATCC 25923 was grown in Tryptone soy broth (TSB) at 37°C for 24h and then diluted into the TSB medium (105cells/mL) supplemented with 2% glucose (w/v). For each concentration of the tested drugs (2–1024μg/mL) and LPE (5–90%), 10mL of bacterial suspension was incubated in a 6-well plate containing round glass cover slips for 24h at 37°C. After adhesion, the non-adhering bacteria were removed by rinsing the substrate four times with sterile PBS. Then the substrate was air dried and observed using AFM. All AFM images were then processed to analyze the biofilm formation behavior.
Statistical analysisData were analyzed using SPSS v. 17.0 software. The Friedman test, followed by the Wilcoxon signed ranks test were used to assess inter-group differences. In addition, statistical significance was set at p-value <0.05.
ResultsAntimicrobial activity of LPEAs presented in Table 1, LPE demonstrated selective antimicrobial properties. Seven S. aureus strains (S3, S4, S5, S6, S1, S8 and S9) and S. aureus ATCC 25923 have a MICs value ranged from 20% to 50% (v/v). The remaining two strains (S2, S7) were more susceptible (MIC 10%). In addition, a variable MBCs (10–90%) (v/v) was observed according to the tested strain (Table 1). On the other hand, out of the nine oral S. aureus strains, eight (Table 1) were considered resistant to BC (MICs between 4 and 128μg/mL) and only one strain (S4) was sensitive (MICs≤2μg/mL); all strains were resistant to CHX (MICs>2μg/mL). Moreover, only one strain (S4) was susceptible to TET (MIC<2μg/mL).
Modulation of drug resistance by LPEData presented in Table 1 showed that the presence of LPE at 1/2-MIC (v/v) in combination with BC resulted in a 4-fold reduction of MICs and MBCs of S. aureus ATCC 25923 and one oral strain (S6). Concerning the synergistic effect of CHX and LPE, there was an important diminution of MIC and MBC of CHX (2–8-fold potentiation) in six strains (Table 1). Furthermore, a 2-8-fold reduction of MIC (μg/mL) of TET against seven strains when combined with LPE was noticed (Table 1).
Biofilm inhibition by LPEBiofilm formation of oral S. aureus strains was evaluated in 96 microtiter wells plate with LPE (ranging from 5% to 90%, v/v). Results were expressed as minimum biofilm inhibition concentration (BIC50 and BIC90). As presented in Table 2, BIC90 of LPE was reached at 54% LPE supplementation, for two oral strains (S4 and S7). While, BIC50 ranged from 28 to 77% (v/v) LPE supplementation (Table 2).
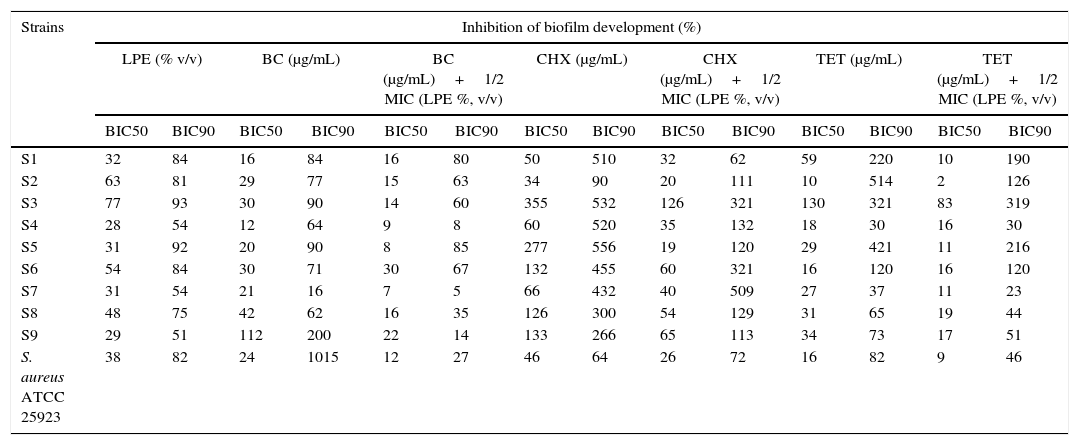
Anti-biofilm effect of Lactobacillus plantarum extract alone or in combination with benzalchonium chloride, chlorhexidine, and tetracycline against oral staphylococci.
| Strains | Inhibition of biofilm development (%) | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| LPE (% v/v) | BC (μg/mL) | BC (μg/mL)+1/2 MIC (LPE %, v/v) | CHX (μg/mL) | CHX (μg/mL)+1/2 MIC (LPE %, v/v) | TET (μg/mL) | TET (μg/mL)+1/2 MIC (LPE %, v/v) | ||||||||
| BIC50 | BIC90 | BIC50 | BIC90 | BIC50 | BIC90 | BIC50 | BIC90 | BIC50 | BIC90 | BIC50 | BIC90 | BIC50 | BIC90 | |
| S1 | 32 | 84 | 16 | 84 | 16 | 80 | 50 | 510 | 32 | 62 | 59 | 220 | 10 | 190 |
| S2 | 63 | 81 | 29 | 77 | 15 | 63 | 34 | 90 | 20 | 111 | 10 | 514 | 2 | 126 |
| S3 | 77 | 93 | 30 | 90 | 14 | 60 | 355 | 532 | 126 | 321 | 130 | 321 | 83 | 319 |
| S4 | 28 | 54 | 12 | 64 | 9 | 8 | 60 | 520 | 35 | 132 | 18 | 30 | 16 | 30 |
| S5 | 31 | 92 | 20 | 90 | 8 | 85 | 277 | 556 | 19 | 120 | 29 | 421 | 11 | 216 |
| S6 | 54 | 84 | 30 | 71 | 30 | 67 | 132 | 455 | 60 | 321 | 16 | 120 | 16 | 120 |
| S7 | 31 | 54 | 21 | 16 | 7 | 5 | 66 | 432 | 40 | 509 | 27 | 37 | 11 | 23 |
| S8 | 48 | 75 | 42 | 62 | 16 | 35 | 126 | 300 | 54 | 129 | 31 | 65 | 19 | 44 |
| S9 | 29 | 51 | 112 | 200 | 22 | 14 | 133 | 266 | 65 | 113 | 34 | 73 | 17 | 51 |
| S. aureus ATCC 25923 | 38 | 82 | 24 | 1015 | 12 | 27 | 46 | 64 | 26 | 72 | 16 | 82 | 9 | 46 |
LEP, Lactobacillus plantarum extract; BIC50, minimum biofilm inhibition concentration that showed 50% inhibition; BIC90, minimum biofilm inhibition concentration that showed 90% inhibition; BC, benzalchonium chloride; CHX, chlorhexidine; TET, tetracycline.
In addition, BC showed various effects on the development of S. aureus biofilms with BIC50 values ranging from 12 to 112μg/mL for the tested strains. Moreover, the combination of BC and 1/2 MIC LPE resulted in a reduction of BIC50 against eight strains (Table 2). In the same way BIC90 was also reduced with the combination of BC and LPE.
Concerning the effect of CHX alone and in combination with LPE, BIC50 values ranged from 34–355μg/mL for CHX for the tested strains (Table 2) whereas the combination of CHX and 1/2 MIC of LPE % (v/v) exhibited a reduction of BIC50 and BIC90 against all the tested strains (Table 2).
Our results revealed that TET may reduce S. aureus attachment to polystyrene (BIC90 reach 30μg/mL for S4). In addition, the combination of TET and 1/2 MIC of LPE exhibited a reduction of BIC50 and BIC90 against nine strains (Table 2).
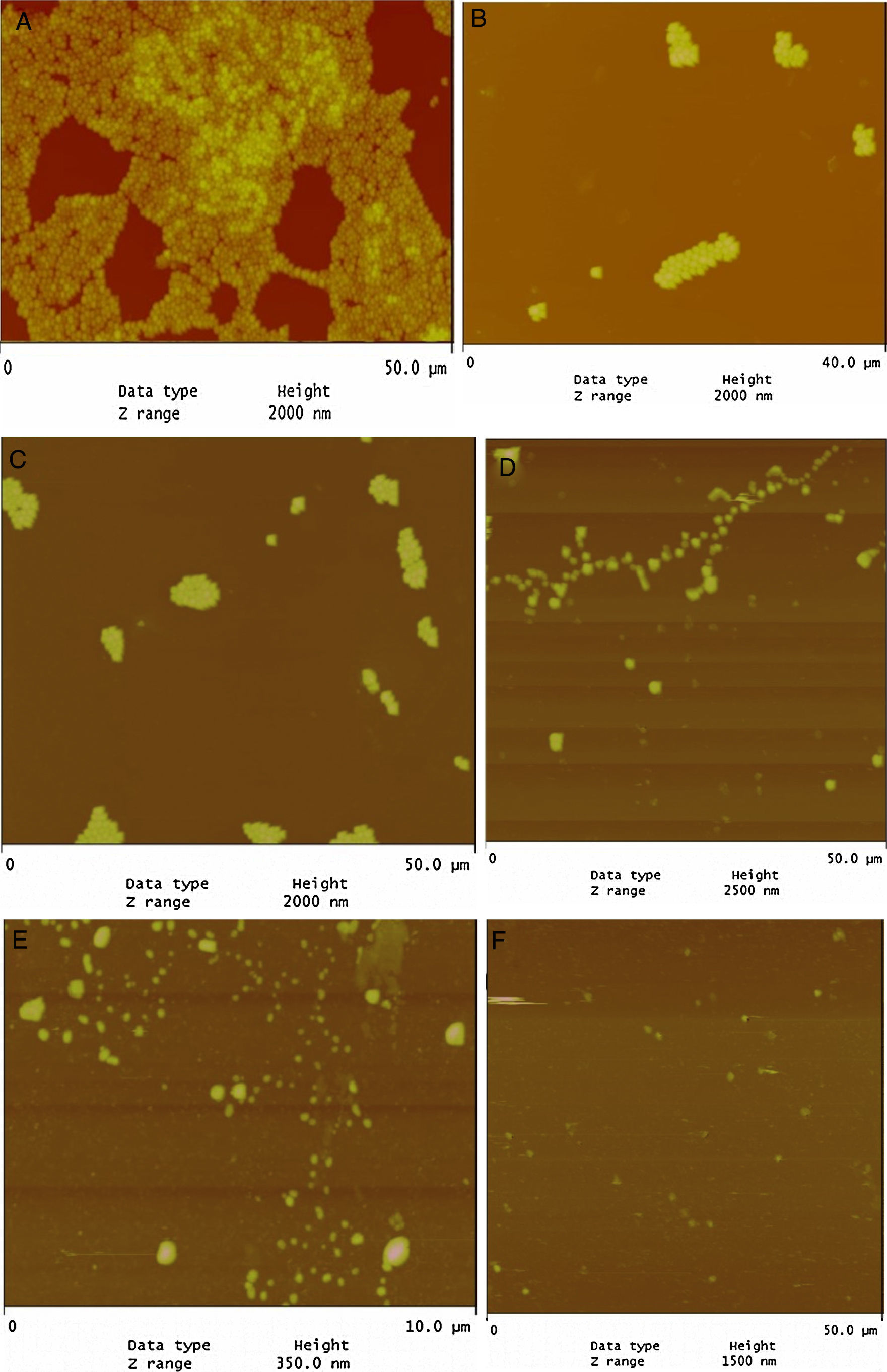
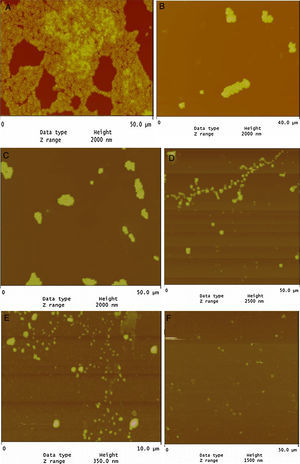
Evaluation of LPE biofilm inhibition by AFMAFM revealed that the tested strains were strongly biofilm positive and had the potency to adhere to glass surface (Fig. 1A). On the other hand, LPE decreased significantly the adherence to glass (Fig. 1D). In addition, the combination of LEP with TET, BC and CHX decreased significantly the biofilm formation (Fig. 1B–E). As presented in Fig. 1F, almost total biofilm inhibition was observed with 16μg/mL of BC combined with 1/2 MIC of LPE % (v/v).
DiscussionA wide ranges of L. plantarum produce anti-microbial peptides with important effects against Gram-positive bacteria.26 Lactic acid bacteria (LAB) are known to have bactericidal activity against pathogenic bacteria. For that, they develop many mechanisms like hydrogen peroxide production causing denaturation of many enzymes and peroxidation of membrane leading to increased permeability.27 Also, the LAB are able to produce bacteriocins which have a bactericidal effect on other species.28 In order to find a natural source able to inhibit pathogenic bacteria and prevent oral biofilm formation, we tested in the effect of LEP on biofilm formation of nine S. aureus isolated from the oral cavity. In addition, we tested the potential effect of LPE to modulate susceptibility of some drugs and disinfectants of 10 S. aureus.
In our study, neutralized LPE supplementation (MIC values 10–50%, v/v) had potential bactericidal effect on oral S. aureus (Table 1). As presented in Table 1, the extract is very effective against S2 and S7 and may constitute a valuable agent to counter the growth of biofilms and undesirable microorganisms on surfaces. Similar results have been found concerning the antibacterial activity of LPE against Vibrio sp.29 and Listeria. monocytogenes.30 Even with sub-inhibitory concentrations (1/2 MIC) LPE showed a potential role to modulate susceptibility of the tested S. aureus. Since BC, TET, and CHX resistance is modulated by efflux pump systems, we suggest that LPE may potentiate, partially, the role of the disinfectant by blocking the respective efflux pump. The efflux pump is the one of major antibiotic resistance mechanisms utilized by bacterial cells to develop resistance. Thus, this resistance mechanism eliminates several classes of antibiotics such as fluoroquinolones, TET, and other compounds such as BC and CHX.
We also noted that the MIC of BC (2–4-fold reduction), CHX (2–8-fold reduction), and TET (2–8-fold reduction) was reduced when the medium was supplemented with LPE at a sub-inhibitory concentration (Table 1). The results showed a statistically significant difference between the antibacterial effects of drugs with and without LPE supplementation (p<0.05). Thus, this finding confirms our hypothesis that the ability to modify susceptibility tested in this study (Table 1) is related to components that have a potential role to inhibit S. aureus efflux pump.
Biofilm formation is an important virulence factor of oral bacteria.5 Our results demonstrate that LPE exhibited a good anti-bioflm activity for all tested strains (Table 2). Similar results were found concerning the anti-biofilm activity of LPE against L. monoctogenes.30 Previous studies have shown that the cells in a biofilm were more resistant to antimicrobial agents compared to free-floating cells.31,32 On the other hand, the anti-biofilm activity of disinfectants and antibiotic (BC, CHX and TET) to polystyrene and glass was enhanced when the medium was supplemented with LPE at different percentages (Table 2 and Fig. 1). The statistical analysis showed a correlation between the percentage inhibition of biofilm of different drugs alone or combined with a sub-MIC of LPE % (1/2 MIC, v/v) (p<0.05). As shown in Fig. 1, AFM demonstrates that S. aureus biofilm were strongly affected by the supplementation of LPE. The identification of anti-biofilm constituents will be essential to be included as alternatives in the control of bacterial biofilms.
The MICs modifying ability of LPE tested in this study may be related to components that have a potential role to inhibit S. aureus efflux pump. Further studies are required to assess their composition and potential clinical relevance.
Conflicts of interestThe authors declare no conflicts of interest.