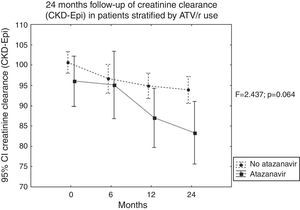
In this study, 275 patients in use of tenofovir were retrospectively followed-up for three years to evaluate risk factors involved in impaired renal function. Analysis of variance (ANOVA) and Tukey's test were used to verify any differences in creatinine levels and estimated clearance at 0, 6, 12, 24 and 36 months, adjusting for the co-variables sex, skin color, age >50 years, arterial hypertension, diabetes and the use of the ritonavir-boosted protease inhibitors (PI/r) lopinavir/r or atazanavir/r. The software package STATISTICA 10® was used for statistical analysis. The patients’ mean age was 43.2±10.7 years. Systemic arterial hypertension (SAH) and diabetes were found in 20.4% and 8.7% of the patients, respectively. Overall, 96.7% were on tenofovir associated with lamivudine (TDF+3TC), 39.3% on lopinavir/r, 29.8% on efavirenz, and 17.6% on atazanavir/r. There was a statistically significant difference in estimated creatinine clearance at 24 months, when the co-variables male (F=3.95; p=0.048), SAH (F=6.964; p=0.009), and age over 50 years (F=45.81; p<0.001) were taken into consideration. Analysis of the co-variable use of atazanavir/r showed a tendency toward an increased risk over time (F=2.437; p=0.063); however, no significant time interaction was seen. At 36-month, a statistically significant difference was found for age over 50 years, (F=32.02; p<0.05) and there was a significant time-by-sex interaction (F=3.117; p=0.0149). TDF was discontinued in 12 patients, one because of a femoral neck fracture (0.7%) and 11 due to nephrotoxicity (4%). Of these latter cases, 9/11 patients were also using protease inhibitors. These data strongly alert that tenofovir use should be individualized with careful attention to renal function especially in male patients, over 50 years, with SAH, and probably those on ATV/r.
For years now, antiretroviral therapy (ART) has been changing the natural history of the human immunodeficiency virus (HIV) infection by reducing its related morbidity and mortality, resulting in high survival rates among infected patients.1,2 However, new adverse effects have arisen, with an important impact on patients’ quality of life such as progressive changes in renal function apparently related to the use of tenofovir (TDF).3 Initiating ART with TDF as a component of a dual-nucleoside analog regimen is already a consensus in the literature due to its potency, good tolerability profile and, mainly, to its low pill burden regimen and co-formulations.4–6 These features increase in importance considering the current context of treating all patients at an early stage,4,5 thus exposing patients to the use of antiretroviral (ARV) therapy for longer periods of time and to the consequent cumulative toxicity,7 without, however, taking into account individual factors that may be associated with greater probability of developing side effects. Another important fact is that TDF is the approved drug for pre-exposure prophylaxis (PrEP),4,6 thereby also generating risks for patients who are not infected with HIV. The objective of the present study was to evaluate the risk factors for developing impaired renal function in patients in use of TDF who were receiving care at an AIDS reference center in Vitória, Espírito Santo, Brazil between April 2006 and April 2013.
Material and methodsPatients infected by HIV [either mono-infected or co-infected with the hepatitis B virus (HBV) or with the hepatitis C virus (HCV)] and HBV mono-infected patients, at the outpatient HIV clinic at Santa Casa de Vitoria, ES, all of whom in use of TDF between April 2006 and April 2013, were included in the study. This outpatient clinic is an important reference center in the state of Espirito Santo, taking care of around 900 individuals. For inclusion in the study, patients’ records should have information about the urine analyses, creatinine measurements and estimated clearance calculated according to the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equation, prior to treatment and at 6 months, 1 year, 2 years, and 3 years of follow-up. A total of 275 patients were screened as they had at least one measure before initiating TDF and one measure after that. As AZT/3TC was the ART backbone recommended by National Guidelines at that time, almost 30% of the patients were using TDF. Of those on TDF, 21 patients were excluded, as no measures before use were available. The CKD-EPI was chosen because it is more frequently used to evaluate renal function in HIV patients.8 An Excel database was created and repeated measures analysis of variance (ANOVA) was used to check for any changes in renal function at 6, 12, 24, and 36 months adjusting for the co-variables sex, skin color, diabetes, systemic arterial hypertension (SAH), age, and use of the ritonavir-boosted protease inhibitors (PI/r), atazanavir/r or lopinavir/r. Repeated-measures ANOVA is an extension of the paired-t test that is required for comparing three or more dependent means. It is used when variables are measured at multiple times in the same subject to assess changes due to an intervention. Tukey's test was used whenever any difference was found. The software STATISTICA 10® was used for statistical analysis. The significance level adopted for the study was 5%.
ResultsOf the 275 participants in the study, 176 (64%) were white, 171 were male (62%). The mean age was 43.2±10.7 years. Overall, 253 were HIV-mono-infected patients, while 10 were co-infected with HBV, 5 with HCV, and 7 were HBV-mono-infected. HIV risk factor was heterosexual relation in 172 cases, while 77 cases consisted of men who had sex with men (MSM), and 12 patients were injection drug users (IDU). Overall, 20.4% had SAH. Fasting glucose levels were abnormal in 14.9%, with 8.7% being diabetics (Table 1). The association of TDF with lamivudine (TDF+3TC) was used by 96.7%. Lopinavir/r was used by 39.3% of patients, efavirenz (EFV) by 29.8%, and atazanavir/r by 17.6%, while 6.9% used another PI/r, and 4.6% of the patients were on nevirapine. At baseline, mean estimated glomerular filtration rate (eGFR) was 100.5±17.66mL/min (n=275 patients). Mean eGFR decreased to 96.15±23.15mL/min at six months (measured in 244 patients, 61.5% male), 94.5±20.74mL/min at one year (measured in 247 patients, 62.3% male), 92.18±22.42mL/min at two years (measured in 227 patients, 62.6% male), and 89.80±21.1mL/min at three years of follow-up (measured in 170 patients, 63.5% male). Proteinuria was detected, using test strips, in 1.1% of patients at baseline, 2.5% at six months, in 3% at one year, and in 5% at two and three years of follow-up. At the 24-month analysis, statistically significant differences were found over time in patients’ creatinine levels and clearance (p<0.05). Male sex (F=3.95; p=0.048), presence of SAH (F=6.964; p=0.009), and age over 50 years (F=45.81; p<0.001) were statistically associated with greater decrease in estimated clearance levels. In addition, there was a trend toward renal function deterioration among those on atazanavir/r (F=2.437; p=0.064) (Table 2 and Fig. 1). Furthermore, the time-by-skin color interaction showed a tendency toward a decrease in the estimated creatinine clearance in non-white individuals (F=2.201; p<0.087). However, there was no significant time interaction effect for any of the co-variables evaluated at 24 months. At 36-month, age over 50 years was the only co-variable associated with significant decrease in the estimated creatinine clearance (F=32.020; p<0.05), with time-by-sex interaction (F=3.117; p=0.015) also being statistically significant. Tukey's test showed a significant difference in impaired renal function in men (Table 3). The drug had to be discontinued in 12 patients, 11 because of nephrotoxicity, characterized by eGFR <60mL/min and/or proteinuria (4%), and one because of a femoral neck fracture (0.7%). Of the 11 patients whose treatment was discontinued because of nephrotoxicity, nine were also using PIs.
Characteristics of HIV mono-infected patients and co-infected with HBV or with HCV at the outpatient HIV clinic at Santa Casa de Vitoria, ES, 2006–2013.
| N° | % | |
|---|---|---|
| Sex | ||
| Male | 171 | 62.2 |
| Female | 104 | 37.8 |
| Skin color | ||
| White | 98 | 35.6 |
| No white | 176 | 64.0 |
| Missing | 1 | 0.4 |
| Age | ||
| 20–30 | 23 | 8.4 |
| 30–40 | 84 | 30.5 |
| 40–50 | 91 | 33.1 |
| 50–60 | 53 | 19.3 |
| Over 60 | 24 | 8.7 |
| Risk factor for HIV infection | ||
| Heterosexual | 171 | 62.2 |
| MSMa | 77 | 28.0 |
| IDUb | 12 | 4.4 |
| Transfusion | 2 | 0.7 |
| Unknown | 13 | 4.7 |
| Arterial hypertension | 56 | 20.4 |
| Diabetes | 24 | 8.7 |
Repeated measures analysis of variance (ANOVA) comparing estimated creatinine clearance (CKD-EPI) evolution in time to male sex, skin color, diabetes, use of PI/r, use of atazanavir, SAH, and age over 50s.
| Time ≤24 months | P-value | |
|---|---|---|
| Factors | F | |
| Time | 10.859 | 0.000 |
| Male sex | 3.950 | 0.048 |
| Time-by-sex | 2.223 | 0.084 |
| Time | 14.772 | 0.000 |
| Skin color | 0.387 | 0.535 |
| Time-by-skin color | 2.201 | 0.087 |
| Time | 9.719 | 0.000 |
| Diabetes | 0.812 | 0.369 |
| Time-by-diabetes | 1.851 | 0.137 |
| Time | 12.405 | 0.000 |
| PI/r | 0.086 | 0.769 |
| Time-by-PI/r | 0.725 | 0.537 |
| Time | 12.495 | 0.000 |
| Atazanavir | 3.199 | 0.075 |
| Time-by-atazanavir | 2.437 | 0.064 |
| Time | 13.180 | 0.000 |
| SAH | 6.964 | 0.009 |
| Time-by-SAH | 0.894 | 0.444 |
| Time | 15.542 | 0.000 |
| Age>50 | 45.814 | 0.000 |
| Time-by-age | 2.183 | 0.089 |
Values in bold show p<0.05.
Tukey's test for multiple analysis for creatinine clearance (CKD-EPI) and time-by-sex interaction.
| Sex | Time (months) | {1} | {2} | {3} | {4} | {5} | {6} | {7} | {8} | {9} | {10} |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Male | 0 | 0.331 | 0.002 | 0.000 | 0.000 | 0.814 | 0.994 | 0.465 | 0.498 | 0.442 | |
| Male | 6 | 0.331 | 0.792 | 0.196 | 0.000 | 1.000 | 1.000 | 0.994 | 0.996 | 0.992 | |
| Male | 12 | 0.002 | 0.792 | 0.995 | 0.166 | 1.000 | 0.984 | 1.000 | 1.000 | 1.000 | |
| Male | 24 | 0.000 | 0.196 | 0.995 | 0.749 | 0.997 | 0.854 | 1.000 | 1.000 | 1.000 | |
| Male | 36 | 0.000 | 0.000 | 0.166 | 0.749 | 0.735 | 0.272 | 0.958 | 0.948 | 0.965 | |
| Female | 0 | 0.814 | 1.000 | 1.000 | 0.997 | 0.735 | 0.992 | 0.999 | 1.000 | 0.999 | |
| Female | 6 | 0.994 | 1.000 | 0.984 | 0.854 | 0.272 | 0.992 | 0.761 | 0.800 | 0.731 | |
| Female | 12 | 0.465 | 0.994 | 1.000 | 1.000 | 0.958 | 0.999 | 0.761 | 1.000 | 1.000 | |
| Female | 24 | 0.498 | 0.996 | 1.000 | 1.000 | 0.948 | 1.000 | 0.800 | 1.000 | 1.000 | |
| Female | 36 | 0.442 | 0.992 | 1.000 | 1.000 | 0.965 | 0.999 | 0.731 | 1.000 | 1.000 |
Values in bold show p<0.05.
After 36 months of TDF use, patients in the present study suffered significant impairment of renal function that worsened over time. Scherzer et al. followed up a large cohort of HIV-infected US veterans comparing individuals who had been exposed to TDF over a 5-year period with controls who had not. Those investigators reported progression to chronic kidney disease in 7.7% of those who had been on TDF compared to 3.8% of those who had not.9 In the D:A:D study, the cumulative use of TDF and atazanavir/r was found to be an independent predictor of eGFR <70mL/min, while an eGFR in the range of 60–70mL/min was also associated with a higher rate of discontinuation of TDF.10 The present study also indicates a trend toward a reduction in the eGFR with atazanavir/r and discontinuation of TDF with an eGFR <60mL/min. A metanalysis of 17 studies involving 10,889 patients followed up for a mean of 48 weeks showed that regimens with TDF were associated with greater renal function impairment compared to regimens that did not include TDF – a mean difference in eGFR of 3.92mL/min (Cockroft-Gault formula; 95% CI: 2.13–5.7mL/min).7 A retrospective cohort study conducted by the Kaiser Permanente Medical Care Program in the United States showed that even small changes in the glomerular filtration rate in absolute terms tend to persist and intensify over time.11 A study conducted with a French cohort also showed that exposure to TDF was associated with more chronic kidney disease, particularly when administered together with PIs (incidence rate ratio: 3.0 with PIs and 1.3 without PIs; p<0.001).12 Even when administered for a short time in pre-exposure prophylaxis (PrEP), a significant decrease in the glomerular filtration rate was found after a four-week exposure to TDF associated with emtricitabine (TDF/FTC) in 2499 HIV-negative patients included in a randomized, placebo-controlled study (−2.4mL/min versus −1.1mL/min; p=0.02, respectively).13
SAH and age >50 years were factors significantly associated with renal function impairment in the present study. Indeed, SAH and age are classic risk factors for deterioration in renal function in the general population.14 This research group has already reported that SAH and age >50 years were risk factors for decreased renal function in HIV-positive patients in the Brazilian state of Espírito Santo.15 A cross-sectional study with 255 patients receiving care at an outpatient clinic in Porto Alegre detected SAH, exposure to TDF, and duration of antiretroviral treatment as risk factors for chronic kidney disease.16 Another cross-sectional study conducted in Rio de Janeiro also associated SAH and exposure to TDF in addition to diabetes and age >50 years as risk factors for eGFR <60mL/min.17 To the best of our knowledge, the present study is the first longitudinal study in Brazil to show progressive loss of renal function, aggravated in a cumulative manner by the duration of exposure to TDF.
In the present cohort, being male was a risk factor for a decrease in eGFR at 36 months of follow-up. We were unable to find any other studies in the literature that supported this finding. Some publications have indicated that being female should be a risk factor for chronic kidney disease, with lower muscle mass being one of the possible explanations for these findings; however, there was no association with the use of TDF specifically.12 In a study involving only women receiving pre-exposure prophylaxis (PrEP), the only difference found between the patients using TDF/FTC orally compared to the use of 1% TDF gel was an increase in creatinine levels (1.3% versus 0.2% p=0.004). Nevertheless, there was no other relevant side effect.18
There are limitations associated with the present study, particularly because it consists of a retrospective analysis of data from patient charts, without a control group of individuals not using TDF. Unfortunately a control group was not feasible, because very few creatinine measures were available for patients on zidovudine, not in sufficient number to compare with patients on TDF. Nevertheless, since the study population is relatively young, the nephrotoxic effect with prolonged use in males, particularly those over 50 years of age and with comorbidities such as SAH, is an important finding. The association with PIs, particularly the tendency to develop nephrotoxicity when atazanavir/r is associated, has been shown in other studies. Widespread treatment with the new co-formulation TDF/3TC/EFV in Brazil, together with the aging of the Brazilian population infected by HIV, demands greater care in monitoring kidney function.
Conflicts of interestThe authors declare no conflicts of interest.