Natural killer cells are a unique type of lymphocytes with cytotoxic capacity, and play important roles against tumors and infections. Recently, natural killer cells have been increasingly valued in their effects in hepatitis B virus infection. Since hepatitis B virus is not cytopathic, the subsequent antiviral immune responses of the host are responsible for sustaining the liver injury, which may result in cirrhosis and even hepatocellular carcinoma. Many studies have confirmed that natural killer cells participate in anti-hepatitis B virus responses both in the early phase after infection and in the chronic phase via cytolysis, degranulation, and cytokine secretion. However, natural killer cells play dichotomic roles: they exert antiviral and immunoregulatory functions whilst contribute to the pathogenesis of liver injury. Here, we review the roles of natural killer cells in hepatitis B virus infection, introducing novel therapeutic strategies for controlling hepatitis B virus infection via the modulation of natural killer cells.
Hepatitis B virus (HBV) infection is one of the most common chronic viral infections in humans. HBV is not cytopathic, i.e. when cellular immune responses are deficient or pharmacologically suppressed, HBV can replicate at high levels in vivo without detectable pathological consequences.1,2 It has been shown that liver injury is in fact due to the antiviral immune response of the host. In chronic HBV infection, prolonged progress may cause persistent liver inflammation, eventually resulting in cirrhosis and hepatocellular carcinoma (HCC).3
It is well known that virus-specific CD8+ cytotoxic T lymphocytes and CD4+ T-helper cells play effective and regulatory roles in anti-HBV immunity.4 However, the roles of innate immunity have triggered extensive debates. Innate immunity is considered to compose the first-line host defense against pathogens, including sensing danger signals, controlling viral replication and dissemination very early after infection, as well as for timely orchestration of virus-specific adaptive responses.5 However, it has been reported that HBV does not modulate host cellular gene transcription, and would therefore induce neither innate antiviral response in hepatocytes nor intrahepatic innate immune responses.6 Furthermore, a portion of chronically infected patients undergo a long-lasting immune tolerance phase that is characterized by a lack of clinical symptoms and high HBV load.7 Hence, the innate immunity is deemed to account for the “absence” of anti-HBV response. Nevertheless, some findings are inconsistent with these conclusions, suggesting an active role of innate immunity in HBV clearance. Natural killer (NK) cells are an essential part of the innate immune system, and participate in the antiviral responses despite the dichotomic characteristics they display.8 Here, we will review the recent studies regarding altered NK cells phenotypes and functions in HBV infection.
General features of NK cellsHuman NK cells are generally defined as a group of lymphocytes that express CD56 but lack the T cell receptor (TCR)–CD3 complex. They represent about 15% of all peripheral blood lymphocytes and this proportion can rise to more than 30% in the liver.9 NK cells exert their effects mainly through the recognition and killing of target cells and the secretion of cytokines such as interferon-γ (IFN-γ) and tumor necrosis factor-α (TNF-α), which can modulate antiviral immune responses. By producing antiviral cytokines and chemokines, NK cells also play an important role in bridging the innate and adaptive immune responses.10 NK cells can be further divided into two subsets, the CD56bright and the CD56dim subsets, according to the cell membrane density of CD56. CD56dim NK cells, expressing high levels of the low-affinity Fcγ-receptor CD16 and the killer immunoglobulin-like receptor (KIR), represents about 90% of all blood NK cells, whilst CD56bright NK cells constitute less than 10% of all blood NK cells and do not express CD16 and KIR.11 Referring to functions, CD56dim NK cells efficiently kill target cells by degranulation but secrete low levels of cytokines; on the other hand, CD56bright NK cells produce a large amount of cytokines upon stimulation but are less cytotoxic. However, CD56bright cells exhibit similar or enhanced cytotoxicity against target cells after prolonged activation compared with CD56dim cells.12 In addition, CD56bright NK cells constitutively express the high- and intermediate-affinity interleukin-2 receptors (IL-2R) and expand in vitro and in vivo in response to low doses of IL-2.9,13,14 On the contrary, resting CD56dim NK cells only express the intermediate-affinity IL-2R and proliferate weakly in response to high doses of IL-2 in vitro, even after induction of the high-affinity IL-2R.9,11,13
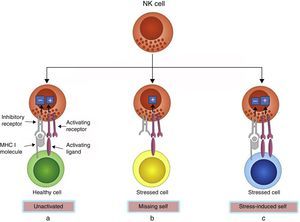
NK cells express an array of receptors including inhibitory and activating receptors, inducing negative and positive signals when combined with their ligands, which can determine whether or not NK cells become activated, enabling them to detect infected or neoplastic cells while sparing normal cells.15 MHC class I molecules, encoded by highly conserved genes inherited independently and expressed by most normal cells, are natural ligands for NK cell inhibitory receptors. Thus, the strength of the activating signals on encountering normal cells is dampened by inhibitory signals, and NK cells are left quiescent. However, MHC class I molecules are often down-regulated on cancerous or virus-infected cells, delivering insufficient inhibitory signals, thus activating NK cells. This is known as the “missing-self” mechanism of NK cell activation. In another condition, NK cells are selectively activated by ‘stressed’ cells, which express upregulated activating ligands for NK cells and thereby overcome the inhibitory signaling delivered by MHC class I molecules. This is known as the “stress-induced self” triggering of NK cell activation16,17 (Fig. 1). In both conditions, NK cell activation leads to degranulation and/or cytokine secretion. In addition, NK cells can be activated upon stimulation by IL-12, IL-15, IL-18 and IFN-α/β.14
Recognition of healthy and stressed cells by NK cells. (a) MHC I molecules are expressed by most normal cells, which transmit inhibitory signals. The strength of inhibitory signals overcomes activating signals, and NK cells are left unactivated. (b) MHC I molecules are often down-regulated on stressed cells, offering insufficient inhibitory signals, thus activating NK cells. This is the “missing-self” mechanism of NK cell activation. (c) Some stressed cells up-regulate activating ligands rather than down-regulate MHC I molecules, which deliver overwhelming activating signals and activate the NK cells. This is the “stress-induced self” mechanism of NK cell activation.
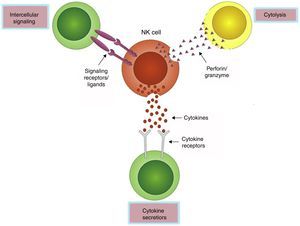
Innate immunity is the first barrier against infection and neoplastic growth, and is distinguished by the rapid participation in the early response to antigens without presensitization. NK cells, as an important element of the innate immunity, display at least three effector functions that contribute to infection control: they can directly kill infected cells by releasing cytolytic granules, induce apoptosis of target cells through crosslinking death receptors and ligands, and produce a variety of immunomodulative cytokines (Fig. 2). Studies have confirmed that NK cells take part in the antiviral responses to certain RNA viruses (e.g. HIV, HCV) and DNA viruses (e.g. CMV).18–20 However, HBV is currently considered as a “stealth” virus and its infection is characterized by a lack of obvious symptoms and liver damage that even lasts for several years. On this account, patients at the very early stage of infection are almost unrecognizable. On the other hand, only chimpanzee and tupaia can be infected naturally by HBV, providing insufficient animal models for its study. Although woodchuck infected with the woodchuck hepatitis virus (WHV) offers a favorable model,21 whether it is an entirely representative of the human setting is yet to be discussed. Mouse models of persistent HBV infections that are analogous to those of human chronic HBV infections have been established,22,23 but with the same problems as with the woodchuck model. To date, taking into consideration these factors, our cognition of the anti-HBV innate immune responses at the earliest presymptomatic stages is still limited.
Some studies suggest that NK cells and even innate immunity do not significantly contribute to the initial control of HBV infection. Studies in chimpanzees and woodchucks have shown that HBV does not induce significant changes in intrahepatic gene expression during entry and reproduction in the liver, and the viral load does not decrease until the onset of the adaptive immune response, several weeks later.24,25 With the development of infection, the clearance of HBV-infected hepatocytes by adaptive immunity correlates with elevated levels of IFN-γ and TNF-α in the liver,2 which could be produced by activated NK cells. However, follow-up experiments indicate that rather than NK cells, CD8+ cells, which are also sources of IFN-γ and TNF-α, are the main effector cells responsible for viral clearance.26
However, some other experiments showed contradictory results. NK cells express mRNAs coding for toll-like receptors (TLR), TLR1 to TLR9, which recognize certain non-self molecules and initiate immune responses.27In vitro, HBV-plasmid DNA promotes NK cell activation including cytotoxicity and IFN-γ production in the liver through TLR/IFN-α-mediated signaling pathways.28 In a study carried out in a high-titer HBV replication mouse model, NK cells were required to eliminate HBV infection, presumably through the induction of HBV-specific CD8+ T cell response.28 Early after infection with high titers of WHV, NK cells were activated and virus load was reduced significantly, suggesting that the innate response is activated in the liver soon after exposure to a certain dose of WHV.29 Owing to the delayed appearance of symptoms or the totally asymptomatic nature of HBV infection, studies of the early immune events in HBV-infected people are rare. A study of two blood donors who were identified by accident to have seroconverted with the appearance of hepatitis B surface antigen (HBsAg) and subsequent rise of HBV viremia without alanine aminotransferse (ALT) elevation, have shown that NK cells are able to mount an early and efficient response to HBV, and that the innate immune system is able to recognize HBV from the beginning of the infection, which probably contributes to the timely induction of adaptive responses.30 In acute hepatitis B, activation receptor-expressing NK cells are preferentially enriched, while the inhibitory receptor-expressing NK cells are reduced.31 Moreover, according to two independent studies, NK cells displayed increased or decreased cytolytic activity and IFN-γ production.31,32 These results reveal that NK cells participate in the early-stage response after HBV infection.
However, most of these studies were carried out in animal models, which may not fully represent the reality in humans. On the other hand, data from human beings are insufficient. Hence, the role of NK cells in the early phase of HBV infection is still to be demonstrated in humans.
Contribution of NK cells to HBV-induced liver injuryIn HBV infection, NK cells always play contradictory roles, as they exert antiviral and immunoregulatory functions whilst contribute to the pathogenesis of liver injury. The apoptosis of HBV-infected hepatocytes is in favor of self-protection as it helps to viral clearance. But in the case of sustained infection, the continuous injury to the liver is the precondition for liver fibrosis and HCC. In the immune-activated stage of chronic hepatitis B (CHB), NK cells skew toward a cytolytic activity, which correlates positively with the severity of liver damage.33 Enhanced cytolytic activity of NK cells can be partly attributed to altered levels of certain cytokines33 and to the recognition between NKG2D and its ligands.34
Other than cytotoxic effectors-mediated liver damage, apoptotic signal transmission is also an important factor causing hepatocyte death. In healthy livers, NK cells express little or no TNF-related apoptosis-inducing ligand (TRAIL) on their surface35 and hepatocytes express minimal TRAIL death-inducing receptors.36 However, in patients with HBV and liver inflammation, TRAIL-expressing NK cells are enriched in the liver and the hepatocytes express upregulated levels of the TRAIL death-inducing receptor, indicating that NK cells may contribute to liver inflammation by TRAIL-mediated death of hepatocytes.37 Besides, after being activated, NK cells may induce massive HBV-infected hepatocyte degeneration through the Fas/Fas ligand interaction,38 and are even involved in the disease progression of HBV-related acute-on-chronic liver failure (ACLF).39 Intrahepatic PD-1/PD-L1 up-regulation is closely associated with the viral load and inflammatory responses, which decreases simultaneously with inflammation remission, implying that the PD-1/PD-L1 system participates in liver injury.40,41 Although NK cell dysfunction is reported to correlate with PD-1 up-regulation,42 direct evidences are lacking to certify that PD-1 alters NK cell function in HBV infection.
NK cells in chronic HBV infectionThe controversial role of NK cells in the acute phase of HBV infection has been underlined above. In chronic infection, NK cells display varying changes in proportion, phenotype and/or function, which differ between studies. The defects in NK cells are reflected in many aspects: (1) the percentages of hepatic and peripheral NK cells are reduced in immune-activated CHB patients, with or without changes in their subsets33,43,44; (2) altered expression of activating or inhibitory receptors on NK cells33,43–46; (3) up-regulation of some molecules with inhibitory effects such as T cell immunoglobulin and mucin domain containing molecule-3 (Tim-3);47 (4) maintained or even enhanced cytolytic activity, which is correlated to the severity of liver injury33,43,46; and (5) impaired capacity to produce cytokines such as IFN-γ and TNF-α.43,44,46,47 Viral load reduction through anti-viral therapy partly restores NK cells numbers, cytokine production and inhibitory receptor expression.43,45,48,49 Although these studies are mostly consistent, there are discordances in some details such as the changes in the expression of some receptors33,43–46 and changes in cytolytic activity. The reasons for these differences are not clear yet, but may be ascribed to ethnic variations, sample size, disease process and severity. Despite inconsistent conclusions between studies, NK cells display intact or even increased cytotoxicity but impaired anti-viral cytokines secretion during chronic HBV infection, and these changes may be partly rectified after viral load reduction.
The factors contributing to NK cell dysfunction were demonstrated by many studies. NK cells express mRNAs for all known TLRs. Through these ligands, NK cells may be directly stimulated to induce the production of IFN-γ.27 A recent study revealed that NK cells can be activated by HBV-based DNA plasmids in a TLR-dependent manner, displaying enhanced NK cell effector functions including cellular cytotoxicity and IFN-γ production.26 However, in the persistent HBV infection phase, NK cells suffer functional disorders that can be restored with viral replication suppression. It can be deduced that during long-lasting virus replication, NK cells are influenced by pathological circumstances induced by the HBV infection.
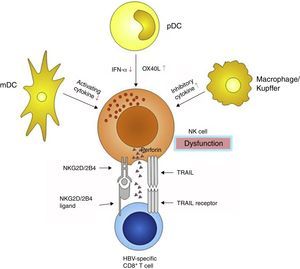
NK cells interact with some other immune cells through receptor–ligand combination and/or regional cytokine secretion. Dendritic cells (DCs) are the most important antigen-presenting cells, and are divided into myeloid dendritic cells (mDC) and plasmacytoid dendritic cell (pDC). DCs bridge the innate and adaptive immunities, and consequently play pivotal roles in eliminating external antigens. During HBV infection, both mDCs and pDCs display functional defects,50–52 which influence the cross talk with NK cells, making inefficient the NK cells activation. mDCs are substantially impaired in their ability to activate NK cells via decreased mDC-derived cytokine secretion, which in turn fail to secrete adequate amounts of IFN-γ.52 pDCs are poor NK activators, potentially through an OX40L/IFN-γ-dependent pathway53,54 (Fig. 3).
Besides DCs, intrahepatic Kupffer cells also participate in the anti-HBV immune response, partly through interfering with the NK cells. Kupffer cells are the main source of the immune-suppressive cytokine interleukin-10 (IL-10), which maintains the humoral immune tolerance during persistent HBV infection55 (Fig. 3). IL-10 acts as an inhibitory cytokine blunting NK activation, which restrains IFN-γ secretion.56 However, another study demonstrated that IL-10 had a significant effect on NK cytotoxicity rather than on IFN-γ or TNF-α production or NK cell activatory receptor expression.57 Blocking the IL-10 pathway may correct defective capacity of NK cells to produce IFN-γ.58 Furthermore, plasma levels of transforming growth factor-β1 (TGF-β1) is also elevated during HBV infection,59 which may impair NK cell-mediated cytotoxic capacity and IFN-γ production by down-regulating the expression of the activating receptors NKG2D and 2B4. Moreover, their intracellular adaptor proteins, respectively DAP10 and SAP, are also decreased.60
Since the immune system is a reticular and syntrophic entirety, NK cell dysfunction also influences the function of the other parts of the system, especially effector T cells. CD8+ T cells are the main effector cells responsible for viral clearance and disease pathogenesis during acute HBV infection.25 However, in the chronic infection stage, they are markedly diminished in number and exhaustive in functions in patients who failed to control virus replication.61,62 The decrease in CD8+ effector T cells can be partly attributed to NK cells, because NK cells cannot only mediate CD8+ T cells death through up-regulation of TRAIL receptors,63 but also limit CD8+ T cells immunity by NKG2D- and 2B4-dependent perforin-mediated lysis64,65 (Fig. 3).
Unfortunately, the restoration of HBV-specific CD8+ T cells is difficult to achieve. Even if a favorable virological response is obtained after anti-viral treatment, HBV-specific CD8+ T cells remain at low levels and express an exhausted phenotype.66,67 This “permanent signature” may result from chronic exposure to HBV-related antigens, which in turn changes the differentiation program of CD8+ T cells. Unlike what is observed in HBV infection, type I interferon can protect anti-viral CD8+ T cells from elimination by NK cell-mediated perforin expression in lymphocytic choriomeningitis virus infection.68 These diverse mechanisms may partly explain why CHB is clinically difficult to manage, with an unsatisfying response rate to IFN treatment. Furthermore, although not yet observed in HBV infection, NK cells may prevent activated CD4+T cells from being killed by the interaction between NKG2A and its ligand.69
NK cells may not be as important as DCs in bridging the innate and adaptive immunities, but their roles are unique, and correlational studies are attracting more and more attention in HBV infection.
NK cells as potential targets for immunotherapyIn the clinical setting, currently available antiviral treatment for CHB infection can be divided into two classes of therapeutic agents: nucleos(t)ide analogs (NAs) and IFN-α. NAs include nucleoside (lamivudine, telbivudine and entecavir) or nucleotide (adefovir and tenofovir) analogs. The major advantages of NAs are good tolerance and potent antiviral activity associated with high rates of on-treatment response to therapy. The advantages of IFN include a finite course of treatment, absence of drug resistance, and an opportunity to achieve a durable response to therapy. Obviously, the advantages of one agent are the disadvantages of the other. Besides, the two agents have been confirmed to contribute little to the restoration of HBV-specific CD8+ T cells. Since current therapies are far from sufficient from economical and effectiveness points of view, novel therapeutic strategies are in great need. It is unclear why individuals respond differently to IFN,70 but it is supposed to be due, at least in part, to NK cell characteristics including cell numbers, receptor expression and function alteration.67,71 Similar results were also obtained with the use of NAs,43,45 indicating that the modulation of NK cell function may help to suppress HBV replication. Aberrant DNA methylation is an early and ubiquitous event during HCC development, and can be detected even in precancerous liver tissues such as chronic hepatitis, liver cirrhosis, or dysplastic nodules.72 A recent study confirmed that this process was closely associated with NK cell activity.73 Hence, regulation of NK cell function is a potential way to treat CHB and to prevent the occurrence of cirrhosis and HCC.
It has been discussed above that NK cell functional alterations include at least three aspects: decreased cytokine secretion, increased cytotoxicity and apoptosis-mediating capacity (Fig. 3). Therefore, we should turn to increasing anti-viral cytokine production and protect other cells from being killed, targeting on the interaction between NK cells’ receptors to their ligands and cytokines in the microenvironment. As the immune system is a complex entity, the influence on every part of this system should be considered to formulate new strategies in order to avoid immune overreaction.
Strategies based on the regulation of NK cell function to treat CHB were only carried out in animal experiments so far. NK cells may achieve enhanced anti-viral viability or alleviated hepatocyte lysis by blockade of some of their activating or inhibitory receptors and ligand interactions.45,74 Increased expression of PD-1 in the liver results in immune disorder, which is to the disadvantage of HBV clearance. PD-1/PD-L1 blockade could reverse immune dysfunction by augmenting IFN-γ secretion and accelerating HBV elimination in vivo.75 IL-10 and TGF-β1 have been shown to restrain NK cells function, and blockade of IL-10 and/or TGF-β1 restored the capacity of NK cells to produce IFN-γ, thereby enhancing their non-cytolytic antiviral capacity.58,60 The contribution of IFN-γ and TNF-α in HBV clearance is undisputed, especially in the acute phase of infection.2 However, in the immunotolerant and immunoreactive phases, elevated levels of IFN-γ and TNF-α are not enough to help eliminating the virus, but allows sustaining the liver injury.76
Concluding remarksThe reason why HBV infection is difficult to manage in the clinical setting is because of the properties of the virus itself, but also of the immune responses of the hosts. As discussed above, the role of NK cells in HBV infection is still controversial: whether NK cells is involved in the early phase of HBV infection, and what alternations have been occurred to NK cells during chronic infection are not known yet. Whereas, it cannot be denied that the persistence of HBV infection and ongoing liver injury could be partly due to the dysfunction of NK cells and subsequent mediation of disorders of the immune system and death of hepatocytes. On account of our limited knowledge, new immunotherapies basing on NK cells are only in the infancy stage. A new subset of human NK cells, denominated as NK-22 cells, was recently discovered.77 They locate in mucosa-associated lymphoid tissues, and are hard-wired to secrete IL-22, which provide protection to hepatocytes78,79 and promote proliferation of liver stem/progenitor cells,80 providing a novel therapeutic candidate for chronic HBV infection.
In summary, on the basis of existing achievements, more researches are still needed to define the exact roles of NK cells in HBV infection. New strategies should be aiming on meliorating the function of NK cells, inhibiting viral replication, alleviating liver injury and avoiding cirrhosis and HCC.
Conflicts of interestThe authors declare no conflicts of interest.
This work was supported by Science Research Project of Twelfth Five-year-Plan “AIDS and Viral Hepatitis Major Infectious Diseases Prevention and Control”, 2012ZX10005004-002 and 2012ZX10005010-002-003, and National Natural Science Foundation of China, 81102570 and 81202662.