Combination antiretroviral therapy (cART) substantially extended the life of people living with HIV (PLHIV). However, prolonged HIV infection and cART increase the risk of comorbidities accelerating age-related muscle, bone, and vascular disorders. This cross-sectional study compared muscle mass and strength, bone mineral density (BMD), and vascular function in middle-aged PLHIV treated with cART vs. non-infected age-matched and older controls.
MethodsAfter careful screening for secondary diseases and medications, body composition, muscular and vascular function were assessed in 12 PLHIV (43.9±8.7 yrs old; HIV-infection for 16.2±8.6 yrs; on cART for 11.6±9.2 yrs), 12 age-matched (CONT, 43.2±8.5 yrs old), and 12 older (OLDER, 74.4±8.3 yrs old) controls through dual x-ray absorptiometry, isokinetic dynamometry, and venous occlusion plethysmography, respectively.
ResultsPLHIV and CONT showed similar relative muscle mass (65.3±8.0 vs. 66.9±7.3%, respectively; P= 0.88) and strength (160.7±53.9 vs. 152.0±52.9 N.m−1, respectively; P= 0.90), which were greater than OLDER (80.6±18.8 N.m−1; P= 0.001). Total BMD was similar in PLHIV (1.04±0.13 g.cm−2) and OLDER (1.00±0.15 g.cm−2, P= 0.86), and both groups presented lower values than CONT (1.20±0.13 g.cm−2, P< 0.01). No significant difference across groups was detected for macrovascular reactivity (P= 0.32).
ConclusionAge-related osteopenia might be accelerated in middle-aged PLHIV on prolonged cART, as their BMD approached values found in older adults. On the other hand, muscle mass, isokinetic strength, and vasodilation capacity were similar in PLHIV and age-matched uninfected controls.
Acquired Immunodeficiency Syndrome (AIDS) is a disease of the immune system due to infection with the Human Immunodeficiency Virus (HIV), leaving the patient vulnerable to life-threatening infections.1 The advent of combination antiretroviral therapy (cART) substantially prolonged the life expectancy of patients, by suppressing viral load and improving immune function with consequent reduction of opportunistic AIDS-related diseases.1
On the other hand, the combination of long-term HIV infection and cART treatment may harm several physiological systems, thereby increasing the risk of non-AIDS related diseases.1,2 In people living with HIV (PLHIV) those morbidities often coexist at an earlier age.3 Given the high prevalence of patients suffering from multimorbidity,3 this has become a major focus of research in HIV settings.2 Indeed, some authors claim that health-care providers should be aware of a potential “accelerated aging syndrome” in PLHIV.4,5 In short, they evoke the possibility that combined effects of HIV infection and treatment-related factors may exceed “normal” aging in the development of health problems. A better understanding of biological decline in PLHIV treated with cART would be crucial to support multidisciplinary interventions to attenuate or reverse those losses.
A recent review using cluster analysis summarized the most common patterns of comorbidities among PLHIV.2 Cardiovascular diseases and musculoskeletal disorders consistently appeared among the most described morbidities. In fact, there is extensive evidence of progressive losses of skeletal muscle mass and strength in PLHIV treated with cART.6-8 In addition, reduced bone mineral density (BMD) and high prevalence of osteoporosis vs. controls have been reported, particularly in women.9,10 Other major changes include body fat redistribution (peripheral lipoatrophy and central accumulation), greater insulin resistance, hyperlipidemia, and vascular dysfunction,1 which are highly prevalent in older patients,11 especially in those with osteoporosis, fracture, and sarcopenia.12
Like aging, HIV infection is associated with pathologies affecting cardiovascular, muscle, and bone health,13,14 which share common molecular and physiological mechanisms, risk factors, and clinical implications.12,15However, we could not find prior studies that concomitantly compared markers of muscle, bone, and vascular health in PLHIV vs. age-matched and older non-infected controls. This would be helpful to test the hypothesis of accelerated reduction in tissue function due to prolonged HIV-infection and cART. In a few words, it would be possible to verify whether those markers are similar in PLHIV and healthy individuals, or if they approach the profile exhibited by older individuals. Therefore, this study compared muscle mass and strength, BMD, and vascular function in middle-aged PLHIV treated with cART vs. age-matched and older non-infected controls. We hypothesized that the combination of prolonged HIV-infection and cART would increase the rates of muscle and bone loss, as well as metabolic changes predisposing the development of vascular dysfunction. Hence, markers of muscle, bone, and vascular health in PLHIV would be closer to values exhibited by older individuals and than to those observed in age-matched non-infected counterparts.
Material and methodsParticipantsParticipants were adults of both sexes aged ≥ 40 yrs. PLHIV should be 40 to 60 yrs-old, being recruited through social media and visits to outpatient clinic from a tertiary hospital at the University of Rio de Janeiro State (RJ, Brazil). Participants allocated in age-matched and older control groups were recruited by means of advertisement in social media and flyers spread at the university facilities. Exclusion criteria for PLHIV consisted in: a) presence of AIDS‐defining illness according to the Centers of Disease Control and Prevention criteria;16 b) use of cART for less than three yrs, time of HIV infection ≤ 5 yrs; c) cerebral toxoplasmosis or any infectious disease affecting the central nervous system; d) regular physical activity within the previous six months; e) neurological and musculoskeletal diseases; f) use of hormonal replacement therapy and other medications with impact on bone and muscle; g) substance abuse (tobacco, alcohol and other drugs). To be eligible for age-matched and older control groups, volunteers were screened for items d, e, f, and g.
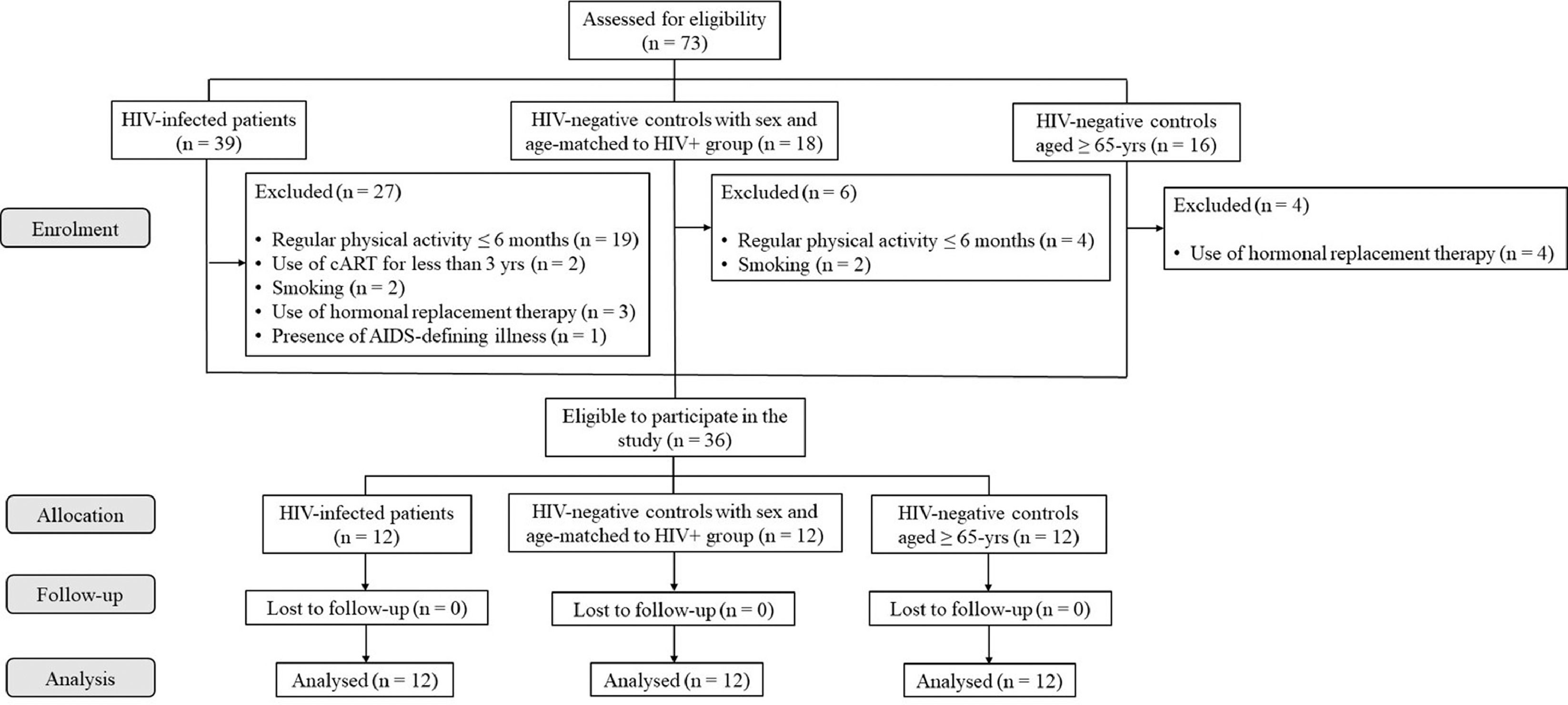
Fig. 1 summarizes the outflow of group selection and allocation. Initially, 39 PLHIV (12 females, 29 to 58 yrs), 18 HIV-negative controls matched for age and sex to PLHIV (14 females, 26 to 58 yrs), and 16 HIV-negative controls aged ≥ 65 yrs (10 females, 65 to 90 yrs) volunteered for the study. After screening for exclusion criteria, 36 volunteers (24 females) were considered eligible and allocated into groups, as follows: a) PLHIV (n=12; 43.9±8.7 yrs; 8 females); b) HIV-negative controls matched for sex and age to PLHIV (CONT, n=12; 43.2±8.5 yrs; 8 females); c) HIV-negative controls aged ≥ 65 yrs matched for sex to PLHIV (OLDER, n=12; 74.4±8.3 yrs; 8 females). There were no dropouts throughout the study.
Study designAfter participants were allocated into groups, they underwent evaluations in two days interspersed with 24 to 48 h intervals between them, always in the morning (7:00–11:00 am). All evaluations were performed by trained technicians blinded for the purposes of the study and group allocation. On the first visit, anthropometric, body composition and isokinetic strength measurements were performed. On the next visit, vascular function was assessed after 6‐hours fasting. Informed signed consents were provided and the study gained approval from the Ethics Committees of the University of Rio de Janeiro State (CAAE 17782513.0.0000.5282) and National Institute of Cardiology (CAAE 42162815.5.0000.5272).
ProceduresBody mass and height were measured by means of calibrated electronic scale FilizolaTM (São Paulo, SP, Brazil) and wall stadiometer SannyTM (São Paulo, SP, Brazil), respectively. Body mass index (BMI) was calculated (Kg/m2). Body fat, lean mass, and bone mineral density were evaluated by dual x-ray absorptiometry (DXA) (DPX-IQ, Lunar Radiation CorporationTM, Madison, WI, USA). Equipment was calibrated according to manufacturer's instructions and scans were performed in high resolution. Principles for body composition analyses with DXA have been described elsewhere.17
Peak torque of lower limbs was assessed using an isokinetic dynamometer (Biodex System 4 Pro, Biodex Medical Systems Inc., Shirley, NY, USA). Participants performed three sets of 10 reps of knee extension and flexion, interspersed with 120-s intervals. Concentric muscle actions were performed by the dominant limb with range of 0–90° and speed fixed at 60°/s. Peak torque and total work were determined and tests were considered as valid when the coefficient of variation was lower than 15%.18 Protocol and test procedures were administered according to previous recommendations.19
Vascular function was evaluated through venous occlusion plethysmography (HokansonTM AI6, Bellevue, WA, USA), after 20 min of rest with subjects in the supine position, as previously described.20 Measurements were performed always in a quiet temperature-controlled room (20–22°C), between 7 to 11 a.m. to minimize circadian effects upon outcomes. During blood flow measurements, venous collecting pressure was set at 50 mmHg to avoid venous return. Wrist cuff occlusion was set at 40 mmHg above systolic blood pressure in order to avoid hand shunt. Forearm blood flow (FBF) at baseline 1, reactive hyperemia to arterial occlusion (post five min forearm arterial occlusion with pressure 50 mmHg above systolic blood pressure), FBF at baseline two and FBF after five min of 0.4 mg sublingual nitroglycerin administration (Nitrolingual BurnsAdlerTM Pharmaceuticals Inc, Charlotte, NC, USA) were measured during two min. Four flow curves were analyzed in baseline 1, baseline 2, and nitroglycerin phases, while the maximum blood flow was recorded in FBF during reactive hyperemia. Percent increase in blood flow during reactive hyperemia was calculated in relation to baseline flow 1 (%HYPER) and its increase after nitroglycerin in relation to baseline flow 2 (%NITRO).
Statistical analysisData normality of major outcomes was ratified by the Shapiro Wilk test and results were expressed as mean ± standard deviation (SD). Categorical variables were compared by chi-square test. One-way ANOVA was applied to test between-group differences regarding clinical characteristics, body composition, muscle strength, and vascular function, followed by Bonferroni post-hoc tests in the event of significant F ratios. All calculations were performed using the GraphPadTM software (Version 6.0, La Jolla, CA, USA) and statistical significance level was set at P ≤ 0.05.
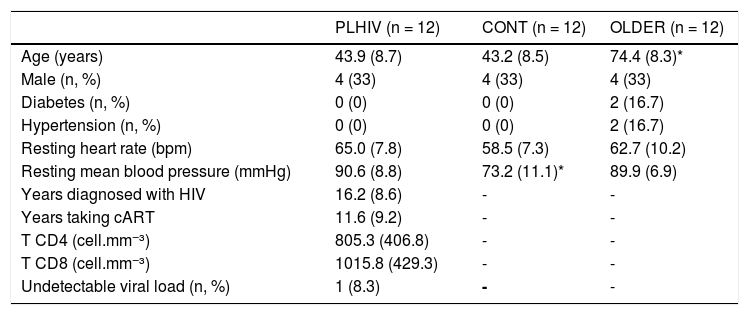
ResultsTable 1 presents the clinical characteristics of the participants. As expected, OLDER was older than PLHIV and CONT (P < 0.001). On the other hand, the three groups were similar regarding all characteristics, except for resting mean blood pressure which was lower in CONT vs. PLHIV and OLDER (P < 0.001). In PLHIV, time ranges of HIV infection and cART intervention were of 5 to 30 years and 3 to 30 years, respectively. As for the cART regimen, 58.3% of patients were using protease inhibitors, 100% nucleoside reverse transcriptase inhibitors, 33.3% non-nucleoside reverse transcriptase inhibitors, and 8.3% integrase inhibitors.
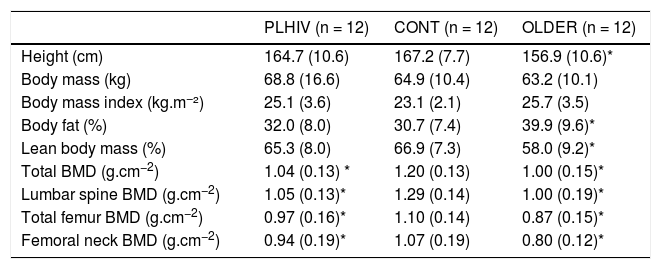
Clinical characteristics of middle-aged people living with HIV (PLHIV) and without HIV age-matched (CONT) and older (OLDER) controls.
| PLHIV (n = 12) | CONT (n = 12) | OLDER (n = 12) | |
|---|---|---|---|
| Age (years) | 43.9 (8.7) | 43.2 (8.5) | 74.4 (8.3)* |
| Male (n, %) | 4 (33) | 4 (33) | 4 (33) |
| Diabetes (n, %) | 0 (0) | 0 (0) | 2 (16.7) |
| Hypertension (n, %) | 0 (0) | 0 (0) | 2 (16.7) |
| Resting heart rate (bpm) | 65.0 (7.8) | 58.5 (7.3) | 62.7 (10.2) |
| Resting mean blood pressure (mmHg) | 90.6 (8.8) | 73.2 (11.1)* | 89.9 (6.9) |
| Years diagnosed with HIV | 16.2 (8.6) | - | - |
| Years taking cART | 11.6 (9.2) | - | - |
| T CD4 (cell.mm−³) | 805.3 (406.8) | - | - |
| T CD8 (cell.mm−³) | 1015.8 (429.3) | - | - |
| Undetectable viral load (n, %) | 1 (8.3) | - | - |
Data expressed as mean (SD). cART, combination antiretroviral therapy.
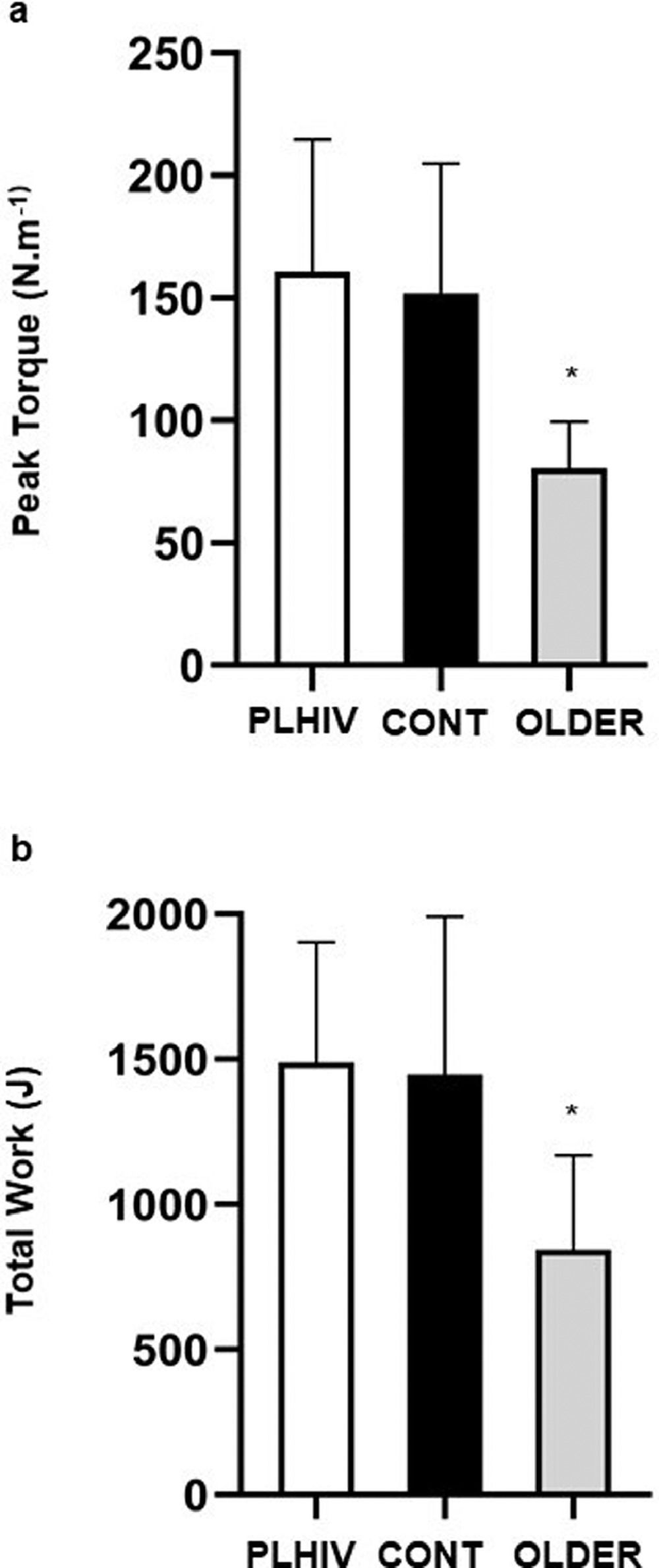
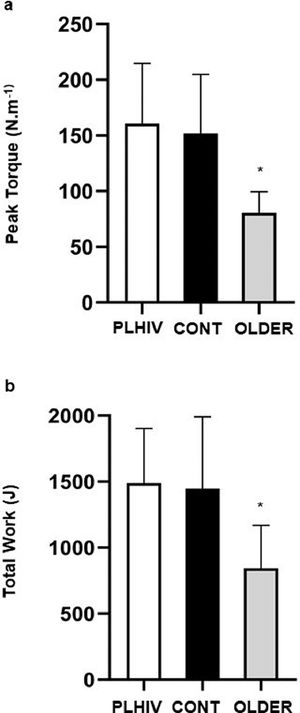
Table 2 depicts data for body composition. Body mass (P = 0.54) and BMI (P = 0.12) were similar across groups. OLDER exhibited higher body fat (P = 0.02) and lower lean mass than CONT (P = 0.02), while these measures were similar between PLHIV and CONT (P = 0.9 and 0.8; respectively). The BMD showed to be more preserved in CONT vs. PLHIV and OLDER (P < 0.01). Fig. 2 exhibits isokinetic strength data. Peak torque and total work were lower in OLDER vs. PLHIV and CONT (P = 0.001).
Body composition of middle-aged people living with HIV (PLHIV) and without HIV age-matched (CONT) and older (OLDER) controls. Data expressed as mean (SD).
| PLHIV (n = 12) | CONT (n = 12) | OLDER (n = 12) | |
|---|---|---|---|
| Height (cm) | 164.7 (10.6) | 167.2 (7.7) | 156.9 (10.6)* |
| Body mass (kg) | 68.8 (16.6) | 64.9 (10.4) | 63.2 (10.1) |
| Body mass index (kg.m−²) | 25.1 (3.6) | 23.1 (2.1) | 25.7 (3.5) |
| Body fat (%) | 32.0 (8.0) | 30.7 (7.4) | 39.9 (9.6)* |
| Lean body mass (%) | 65.3 (8.0) | 66.9 (7.3) | 58.0 (9.2)* |
| Total BMD (g.cm−2) | 1.04 (0.13) * | 1.20 (0.13) | 1.00 (0.15)* |
| Lumbar spine BMD (g.cm−2) | 1.05 (0.13)* | 1.29 (0.14) | 1.00 (0.19)* |
| Total femur BMD (g.cm−2) | 0.97 (0.16)* | 1.10 (0.14) | 0.87 (0.15)* |
| Femoral neck BMD (g.cm−2) | 0.94 (0.19)* | 1.07 (0.19) | 0.80 (0.12)* |
Data expressed as mean (SD). BMD, bone mineral density.
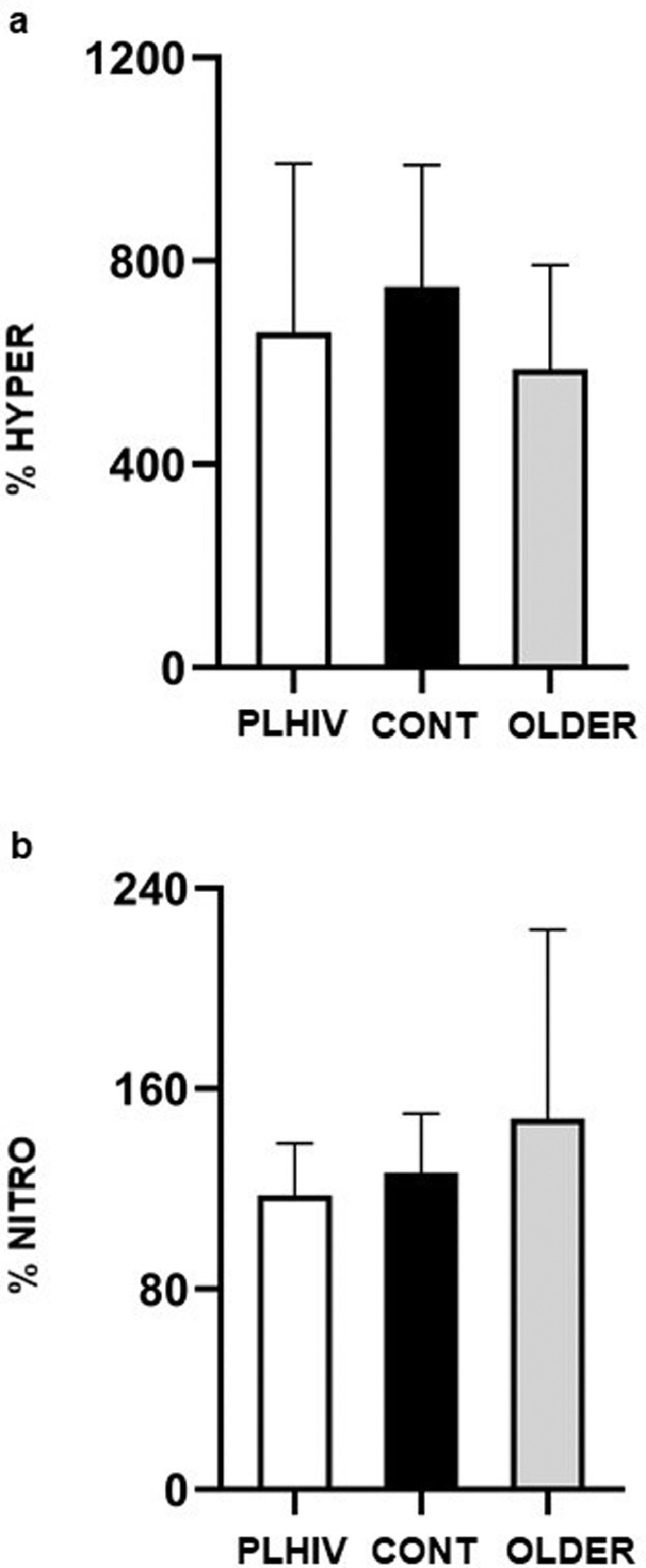
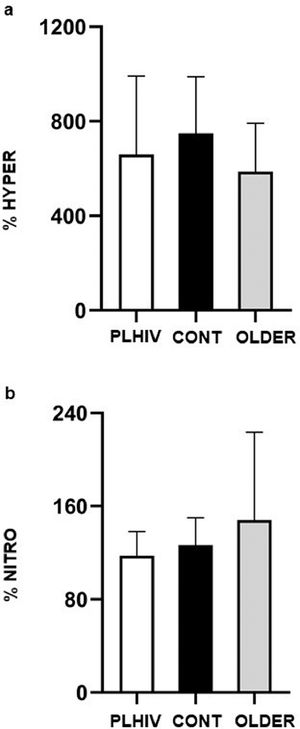
As for vasoreactivity analyses, no difference between PLHIV, CONT, and OLDER was detected for FBF at baseline 1 (2.48 ± 1.26 vs. 2.00 ± 0.67 vs. 2.11 ± 1.17 ml.min−1.100 ml−1, respectively; P = 0.63), after five min of arterial occlusion (15.90 ± 9.12 vs. 14.70 ± 5.60 vs. 10.74 ± 3.50 ml.min−1.100 ml−1, respectively; P = 0.14), at baseline 2 (1.54 ± 0.67 vs. 1.68 ± 0.63 vs. 1.63 ± 0.87 ml.min−1.100 ml−1, respectively; P = 0.94), and after sublingual nitroglycerine (1.85 ± 1.01 vs. 2.07 ± 0.67 vs. 2.04 ± 0.83 ml.min−1.100 ml−1, respectively; P = 0.87). Moreover, as shown in Fig. 3, no significant differences between groups were detected for %HYPER (P = 0.32) and %NITRO (P = 0.47).
Percent increase of forearm blood flow during post-occlusive reactive hyperemia related to baseline flow 1 (%HYPER) and after nitroglycerin in relation to baseline flow 2 (%NITRO) in people living with HIV (PLHIV, n=12), without HIV age-matched (CONT, n=12) and older (OLDER, n=12) controls. Values expressed as mean ± SD.
This cross‐sectional study compared muscle mass, strength, BMD, and vascular function in PLHIV treated with cART vs. age and sex-matched HIV-negative individuals and sex-matched older controls. The main findings were: a) PLHIV exhibited similar muscle mass, isokinetic strength, and vascular function vs. age-matched controls, and greater values vs. older controls; b) On the other hand, BMD in lumbar spine, total femur and femoral neck in PLHIV approached values of older individuals, being significantly inferior in comparison to age-matched healthy controls.
Muscle mass and strength losses are recognized to occur in PLHIV,21,22 being strong contributors to early frailty and disability.23 Long-term effects of viral infection combined with cART toxicities seem to increase the risk of sarcopenia.6,7 However, age-related decline rates in PLHIV remain unclear6,8,24and some prior research even suggested that losses in skeletal muscle mass may be equivalent among PLHIV and non-infected adults over 40 years.8
Several physiological mechanisms contribute to the loss of muscle mass, such as mitochondrial dysfunction, neuro-degenerative diseases, endocrine changes, physical inactivity, and inadequate intake or malabsorption of nutrients.25 In PLHIV, the specific adverse effects of viral infection and cART cannot be disregarded as additional factors potentiating muscle catabolism.26,27 However, in the present study differences in the percentage of lean body mass and strength were not detected in PLHIV compared to age-matched controls. The relatively young age of participants in PLHIV and CONT (43 to 44 yrs) may help to explain these findings. Although the mechanisms underlying the development of premature aging syndromes among PLHIV are multifactorial, chronic immune activation and long-term persistent inflammation seem to play a key role in muscle mass and function losses.23 In this case, differences in muscle mass and function between PLHIV and non-infected individuals would be detected only in further ages.21,22 Evidently, additional research in older PLHIV is warranted to ratify this premise.
As for bone health, greater rates of osteopenia have been reported in PLHIV treated with cART vs. general population of similar age.28 Although the causes of accelerated loss in skeletal mineralization in PLHIV are not fully understood, they seem to include factors such as the use of cART (particularly protease inhibitors), low CD4 count, and the presence of chronic inflammation,9,29 in addition to other traditional risk factors, such as endocrine changes, low skeletal muscle mass, physical inactivity, nutritional deficiencies (calcium and vitamin D), smoking, and alcohol consumption.30 Actually, a meta-analytic review10 showed that the prevalence of osteoporosis among PLHIV would be more than three-fold greater vs. non-infected individuals. Moreover, cART and protease inhibitor treated patients had increased odds for reduced BMD and osteoporosis. Guerri-Fernandez et al.,31 for instance, reported a strong association between HIV infection and hip fracture incidence, with a five-fold risk increase in PLHIV vs. non-infected controls.
Based on these data, one could speculate that PLHIV might have greater risk to develop osteopenia than sarcopenia. Although tempting, this assumption is not possible because we did not assess the incidence of osteopenia and sarcopenia in our sample. However, it is worthy to notice that in the Multicenter AIDS Cohort Study (MACS)32 sarcopenia was present in 41% (76/185) of men living with HIV vs. 36% (67/186) (P = 0.32) of non-infected individuals, while osteopenia/osteoporosis was detected in 16% (30/185) vs. 9% (17/186) (P = 0.003), respectively. This corresponds to a greater risk ratio for osteopenia/osteoporosis (∼1.77) than for sarcopenia (∼1.13). Larger epidemiological studies calculating the risk ratio for both osteopenia and sarcopenia among PLHIV are certainly needed to confirm this premise.
As hypothesized, our data showed that BMD among PLHIV was similar compared to older adults, and lower than values observed in age-matched healthy controls. The BMD presently found in OLDER concurs with values previously reported in the elderly.33,34 To date, most studies on bone density were restricted to young and middle-aged PLHIV vs. age-matched non-uninfected counterparts, while comparative studies with older individuals are scarce.10 Therefore, our data contribute to the current knowledge by ratifying the premise that prolonged infection and cART may accelerate BMD loss in PLHIV, by means of direct comparisons with age-matched and older controls. These findings suggest that strategies to prevent early osteopenia, as hormonal replacement, calcium supplementation, or resistance training, should be considered in middle-aged patients treated with prolonged cART intervention.9,14
Our hypothesis of early vascular aging in PLHIV was not supported by vasoreactivity responses to venous occlusion (endothelial dependent vasodilation), and sublingual nitroglycerine (endothelial independent vasodilatation). Previous evidence have demonstrated that vascular dysfunction, which is a precursor of atherosclerosis and cardiovascular events, is common among PLHIV treated with cART35-37 and older adults.38 The complex interaction of traditional cardiovascular risk factors,39 exposure to cART,40 and HIV infection41 appear to predispose HIV-population to vascular dysfunction. Albeit the lack of statistical significance, the somewhat large difference between PLHIV and age-matched counterparts and the proximity of values exhibited by OLDER for %HYPER suggest that our findings could have been underpowered, due to the relatively small number of subjects or large inter-individual variability of vascular markers. Future research with larger groups are warranted to provide more conclusive information on early vascular aging in PLHIV.
Some limitations of the study must be mentioned. The careful screening of participants to avoid possible biases due to secondary diseases or medications apart from cART hindered the recruitment of a larger sample of PLHIV. As a result, a stratified analysis by sex could not be performed to dismiss sex-related bias. However, the fact that all groups were matched for sex minimized the influence of this factor in our results. In addition, the cross-sectional design is always problematic in establishing age-related effects upon biological variables. Longitudinal studies would provide useful insights of the relationship between secondary effects of HIV-infection and cART and the development of diseases, as well as to avoid cohort effects when describing changes in muscle mass, strength, BMD and vascular function over prolonged periods. Finally, the inclusion of inflammation and oxidative stress biomarkers would allow a better understanding of the hypothetical “accelerated aging syndrome” in PLHIV.
In conclusion, middle-aged patients with prolonged exposure to HIV infection and cART (3 to 30 years) showed preserved muscle mass, strength, and vascular function vs. age-matched non-infected controls. On the other hand, their BMD was lower vs. HIV-negative counterparts and similar to values of non-infected older individuals. Additional comparative studies with larger samples of infected and non-infected individuals, and including patients with longer exposure to HIV infection and cART are warranted to confirm these findings.
Authors’ contributionsConception and design of the study: KGL, GOL, GAP and JPB. Acquisition, analysis, or interpretation of data: KGL, DAB, GOL, GAP and JPB. Drafting the work or revising it critically for important intellectual content: KGL, PF, RBO, DAB, EB and JPB. All authors read and approved the final manuscript.
This work was supported by the National Council for Technological and Scientific Development (CNPq, process 303629/2019-3, recipient PF) and Carlos Chagas Filho Foundation for Research Support in the State of Rio de Janeiro (FAPERJ, process E-26/110.184/2013, recipient PF, E-26/202.720/2019, E-26/010.100935/2018 recipient JB and E-26/111.792/2013 recipient DB).