Acinetobacter spp. are one of the main pathogens responsible for healthcare-associated infections and are associated with high rates of morbidity and mortality globally, mainly because of their high capacity to present and develop resistance to antimicrobials. To identify species of the Acinetobacter and their resistance profiles from samples collected from hospitalized patients, health professionals and hospital environmental sources in the intensive care units of different public reference hospitals in Porto Velho City, Rondônia, Western Brazilian Amazon. Isolates were identified using microbiological and molecular techniques. The antimicrobial susceptibility profile was determined by disk diffusion. A total of 201 Acinetobacter spp. isolates were identified, of which 47.3% originated from hospital structures, 46.8% from patients and 6% from healthcare professionals. A. baumannii and A. nosocomialis were the most prevalent, with frequency of 58.7% and 31.8%, respectively. Regarding the susceptibility profile, it was observed that 56.3% were classified as multidrug-resistant and 76.2% of the samples belonging to A. baumannii were resistant to carbapenems. In contrast, 96.9% were susceptible to polymyxin B and 91.3% to doxycycline. The data presented here can be used to guide and strengthen the control of multidrug-resistant infections caused by Acinetobacter spp., in addition to improving providing information from a traditionally unassisted region of Brazil.
Antimicrobial Resistance (AMR) is defined as the ability of a microorganism to resist the action of an antimicrobial agent designed to kill it and is considered a serious threat to global public health. It is estimated that if no action is taken, AMR could cause the death of approximately 10 million people worldwide by 2050.1 According to The Lancet, there were an estimated 1.27 million deaths attributable to resistance in 2019, and six pathogens (Escherichia coli, Staphylococcus aureus, Klebsiella pneumoniae, Acinetobacter baumannii, and Pseudomonas aeruginosa), were responsible for 929 deaths associated with AMR.2
AMR has caused great concern, especially in hospitals, because patients receiving healthcare or those with weakened immune systems are often at a higher risk of contracting an infection. In addition, antibiotic-resistant bacteria can spread within and between healthcare facilities and cause infections in patients, known as Healthcare-Associated Infections (HAIs), or disseminate into the community or environment (soil and water).3 HAIs are a major public health challenge because their incidence can lead to increased length of stay, hospital costs, high morbidity and mortality rates, and AMR.4
The World Health Organization (WHO) estimates that 15 of 100 patients in low- and middle-income countries will acquire at least one HAI. In developed countries, approximately 30% of patients admitted to Intensive Care Units (ICUs) are affected by HAIs, and this may be 2–3 times higher in underdeveloped countries.5
The genus Acinetobacter is one of the main causes of HAIs and the five species that make up the Acinetobacter baumannii-calcoaceticus complex (A. baumannii. A. nosocomialis, A. pitti, A. seifertii and A. dijkshoorniae) are the most clinically relevant microorganisms.6,7 They are a frequent cause of outbreaks and are responsible for infections reported in ICUs, including sepsis, ventilator-related pneumonia, urinary tract infections, and surgical site infections.8 In the United States, in 2017, Carbapenem-Resistant Acinetobacter caused an estimated 8500 infections in hospitalized patients.9 In Europe, according to the ECDC, it is among the 10 most frequently isolated microorganisms in ICU-acquired bloodstream infection.10 In Brazil, it was the second most isolated microorganism in bloodstream infections in adults ICUs.11
Acinetobacter spp. are typically free-living saprophytes found nearly everywhere and are commonly distributed in the environment. They can be located in soil, water, sewage, humans, food, and animals. Furthermore, these bacteria can survive for long periods on dry surfaces under nutrient-limited conditions, thereby facilitating their persistence and transmission in both natural and hospital environments. Patients on mechanical ventilation, especially those with prolonged duration of hospitalization or ICU stay, have a greater degree of exposure to infected or colonized patients in the hospital and have an increased risk for the acquisition of Multidrug-Resistant (MDR) strains.12
The emergence of Acinetobacter spp. is due to their bacterial dissemination, a consequence of low adherence to infection control measures, especially MDR strains to antimicrobials, particularly carbapenems, which are the antibiotics of choice for treating infections caused by these pathogens.13-15
The most prevalent mechanism of resistance in Acinetobacter is the production of β-lactamases.10 Furthermore, members of the genus Acinetobacter tend to quickly develop resistance to antimicrobial agents, are intrinsically resistant to many antibiotics, and can acquire new resistance mechanisms, corroborating the high dissemination of MDR microorganisms, Extensive Drug Resistance (XDR), and pan-drug resistance.11
Thus, in 2017 the WHO classified Carbapenem-Resistant Acinetobacter Baumannii (CRAB) as a critical priority for the discovery and development of new antibiotics. In Brazil, CRAB is highly prevalent, and the rates of infection by this pathogen have been progressively increasing, presenting relative incidence characteristics that vary according to hospital setting, geographical area, and intervention of hospital services.12,13
Therefore, the execution of projects regarding AMR is essential, especially in the Amazon region of Brazil, in which there are few studies aimed at the identification of Acinetobacter and its profile of AMR in the hospital environment, especially the ICU. This study aimed to identify species of the genus Acinetobacter and their resistance profiles from samples collected from hospitalized patients, health professionals, and hospital environmental sources in the ICU of different public reference hospitals in Porto Velho City, Rondônia, Western Brazilian Amazon, between 2017 and 2019.
MethodsSpecimen collection and bacteriologyClinical samples were collected from patients (oral cavity, armpit, tracheostomy secretion, wound secretion, urine, and blood) admitted to the ICU and health professionals (swab secretion of nasal mucosa and nails) of four reference hospitals in the public network of Porto Velho City, Rondônia, Brazil, named A, B, C, and D, between 2017 and 2019.
Swab samples from hospital environments, including beds, mechanical ventilation devices, computer keyboards, sinks, floors, operating room machines, water taps, and stretches, were collected from the ICU.
Samples were transported to the microbiology laboratory of the Oswaldo Cruz Rondônia Foundation within two hours of processing.
All clinical samples were seeded in blood agar (AS; HiMedia, India), McConkey (MC; Neogen, Brazil), chromogenic (CA; BD, Alemanha), and methylene blue eosin (EMB; HiMedia, India) media and incubated aerobically at 37±2 °C for 18–24 h.
The swabs from hospital structures and health professionals were cultured in Luria-Bertani Broth (LB; Kasvi, Italy) for 18–24 h at 37±2 °C, in an orbital shaker at a speed of 100 rpm and subsequently seeded on AS, CA, and EMB agar plates. All the colonies suspected to be Acinetobacter spp. were subjected to molecular identification.
Species identificationThermal shock was used to extract genomic DNA from the suspected Acinetobacter spp.14 PCR amplification of bacterial ribosomal DNA using primers 16SF (GYCCADACWCCTACGG) and 16S08R (CACGAGCTGACGAC) was performed for bacterial identification.15 A negative control for was prepared using all reagents except the DNA template, and genomic DNA from E. coli O42 was used as a positive control. The amplicons were purified using a QIAquick Gel Extraction kit (Qiagen, Germany) according to the manufacturer's instructions.
Amplicons were quantified using a NanoDrop1000 (Thermo Fisher Scientific, Waltham, MA, USA). Sequencing was performed by the Sanger method on an ABI 3100 Prism DNA Sequencer (Applied Biosystems, Waltham, MA, USA). The sequences were treated using BioEdit Sequence Alignment Editor software (version 7.0) with a Phred quality score of 30. A consensus sequence was generated for each amplicon from each isolate. Species were identified by aligning the consensus sequences with a Basic Local Alignment Search Tool (BLAST) database.
Antimicrobial susceptibility testingAntimicrobial susceptibility tests were performed using the disk diffusion method on Mueller-Hinton agar (KASVI) and commercial antimicrobial disks (Oxoid, UK) according to the guidelines of the Clinical and Laboratory Standards Institute (CLSI).16 The antibiotics tested were distributed into the following classes: quinolones (Levofloxacin [LEV 5 μg], Ciprofloxacin [CIP 5 μg]); Cephalosporins (Ceftriaxone [CRO 30 μg], Cefepime [FEP 30 μg], Cefotaxime [CTX 30 μg], Ceftazidime [CAZ 30 μg]); Carbapenems (Imipenem [IPM 10 μg], Meropenem [MEM 10 μg]); β-lactamase inhibitors (Ampicillin/Sulbactam [SAM 10/10 μg], Piperacillin/Tazobactam [TZP 110 μg; aminoglycosides (Amikacin [AMK 30 μg], Tobramycin [TOB 10 μg], Gentamicin [GEN 10 μg]) and tetracyclines (Doxycycline [DO 30 μg], Tetracycline [TET, 30 μg]).
For the susceptibility test to polymyxin B, a microdilution system was used to determine the Minimum Inhibitory Concentration (MIC) (Policimbac, Probac, Brazil), according to the manufacturer's instructions. E. coli ATCC 25922 and P. aeruginosa ATCC 27853 were used as quality control strains in all tests.
For MDR classification of Acinetobacter spp. isolates, the criteria described by Magiorakos et al. (2012) was used, which determines MDR as those isolates resistant to at least one representative of three or more classes of antibiotics.17
Statistical analysisFor data analysis, BioEstat 5.0 and Jamovi 1.8 software were used. Chi-square and Fisher's exact tests were used to analyze the nominal qualitative variables. Ordinal qualitative variables were analyzed using the Kruskal-Wallis test. When statistical significance was demonstrated, comparisons between groups were analyzed using the Tukey, Dunn, or Student-Newman-Keuls tests. The Shapiro-Wilk test was used for normality distribution. To measure the effect, an Odds Ratio (OR) test was used.
All results with a p-value ≤ 0.05 were considered statistically significant.
Ethical considerationsThis study was approved by the Ethics Committee of the Tropical Medicine Research Center (Process n° 2.368.951).
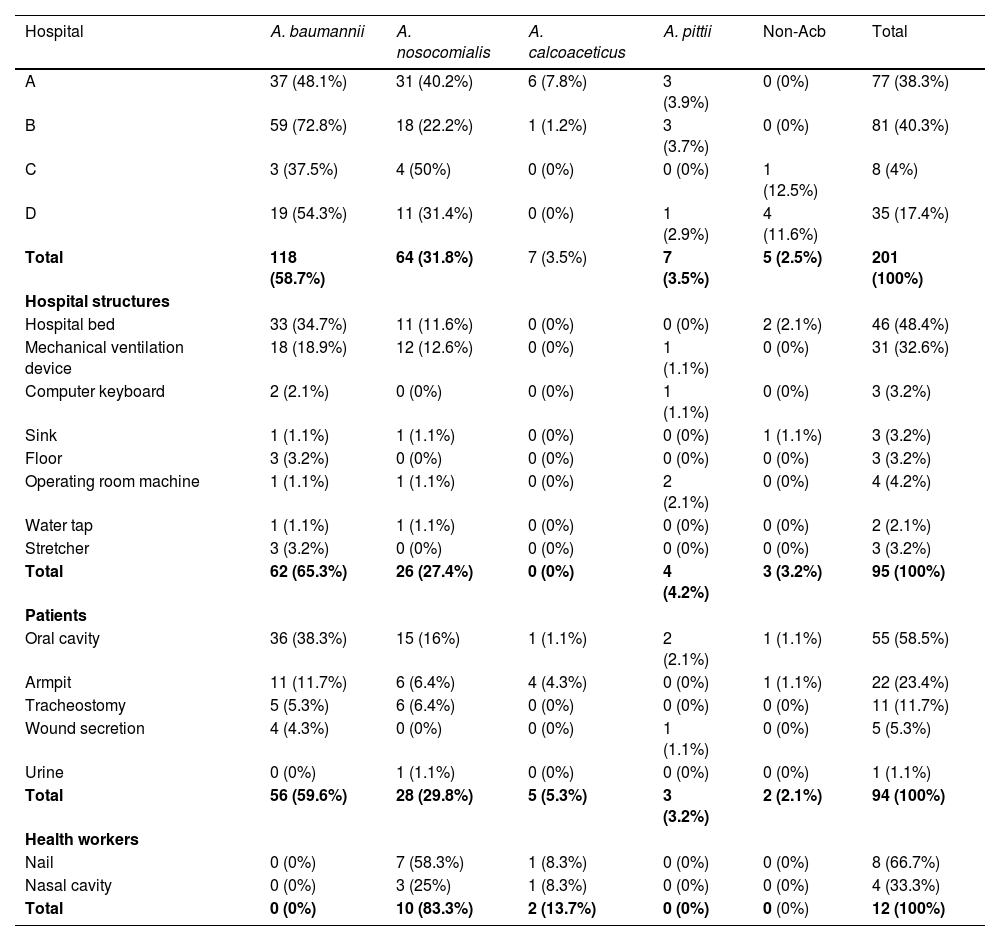
ResultsIn the present study, 201 isolates of Acinetobacter spp. were identified, with 47.3% (95/201) originating from hospital structures, 46.8% (94/201) from patients, and 6% (12/201) from healthcare professionals. Table 1 shows the frequencies of each Acinetobacter spp. identified at each hospital and collection site. A. baumannii and A. nosocomialis were the most prevalent bacteria in the ICUs of the four hospitals studied, with 58.7% (118/201) and 31.8% (64/201), respectively. Other species were also identified such as A. calcoaceticus and A. pittii, both comprising 3.5% (7/201) of the isolates.
Prevalence of Acinetobacter spp. species among Hospitals and collection sites.
Non Acb, A. junii; A. seifertii; A. variabilis; A. radioresistens.
Similarly, A. baumannii and A. nosocomialis were also frequently found in hospital structures, with percentages of 65.3% (62/95) and 27.4% (26/95), respectively, and were isolated mainly from beds and mechanical ventilation devices. A. baumannii was isolated in 59.6% (56/94) of the patient samples, with the oral cavity being the site of greater colonization (38.3%, 36/94), followed by the axillae (11.7%, 11/94), and tracheostomy (5.3%, 5/94). In samples from healthcare workers, A. nosocomialis was the most prevalent species, representing 83.3% (10/12) of the isolates, followed by A. calcoaceticus at 13.7% (2/12).
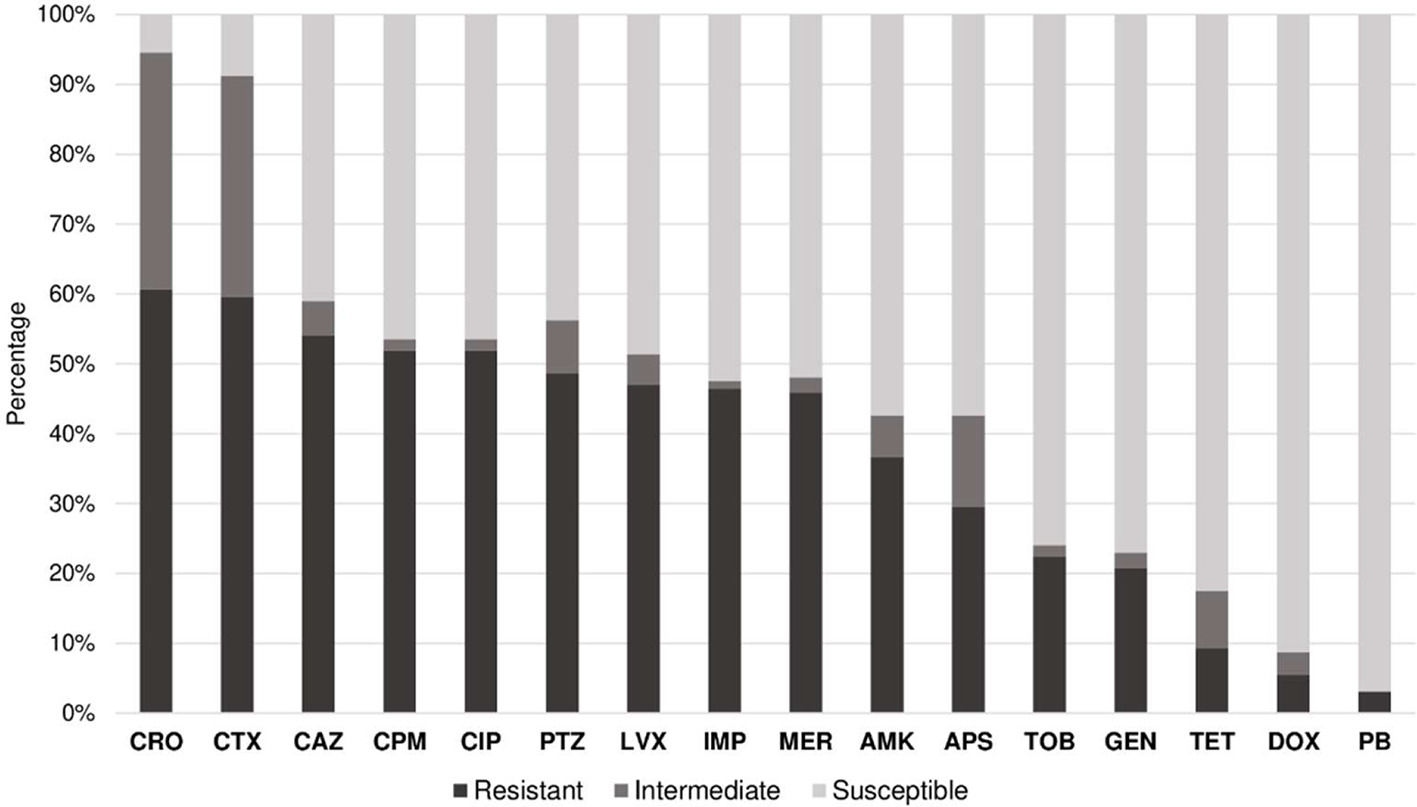
Of the 201 isolates identified, it was possible to evaluate the antimicrobial susceptibility profile of 183 due to the loss of viability in some samples during the study period. We observed that 60.7% (111/183) of the samples were resistant to ceftriaxone, 59.6% (109/183) to cefotaxime, 54.1% (99/183) to ceftazidime, and 51.9% (95/183) to cefepime. Furthermore, 76.2% of the samples (80/105) belonging to A. baumannii were resistant to carbapenems and 56.3% (103/183) of the evaluated samples were classified as MDR.
In contrast, 96.9% (156/183) of the isolates were sensitive to polymyxin B, 91.3% (167/183) to doxycycline, 82.5% (151/183) to tetracycline, 77% (141/183) to gentamicin, 75% (139/183) to tobramycin, 57.4% (105/183) to ampicillin-sulbactam, 52.5% (96/183) to imipenem, and 51.9% (95/183) to meropenem (Fig. 1).
Susceptibility profile of Acinetobacter spp. against antimicrobials. Notes: APS, Ampicillin-sulbactam; PTZ, Piperacillin-tazobactam; CAZ, Ceftazidime; CPM, Cefepime; CTX, Cefotaxime; CRO, Ceftriaxone; MER, Meropenem; IPM, Imipenem; GEN, Gentamicin; TOB, Tobramycin; AMK, Amikacin; DOX, Doxycycline; TET, Tetracycline; CIP, Ciprofloxacin; LVX, Levofloxacin; PB, Polymyxin B.
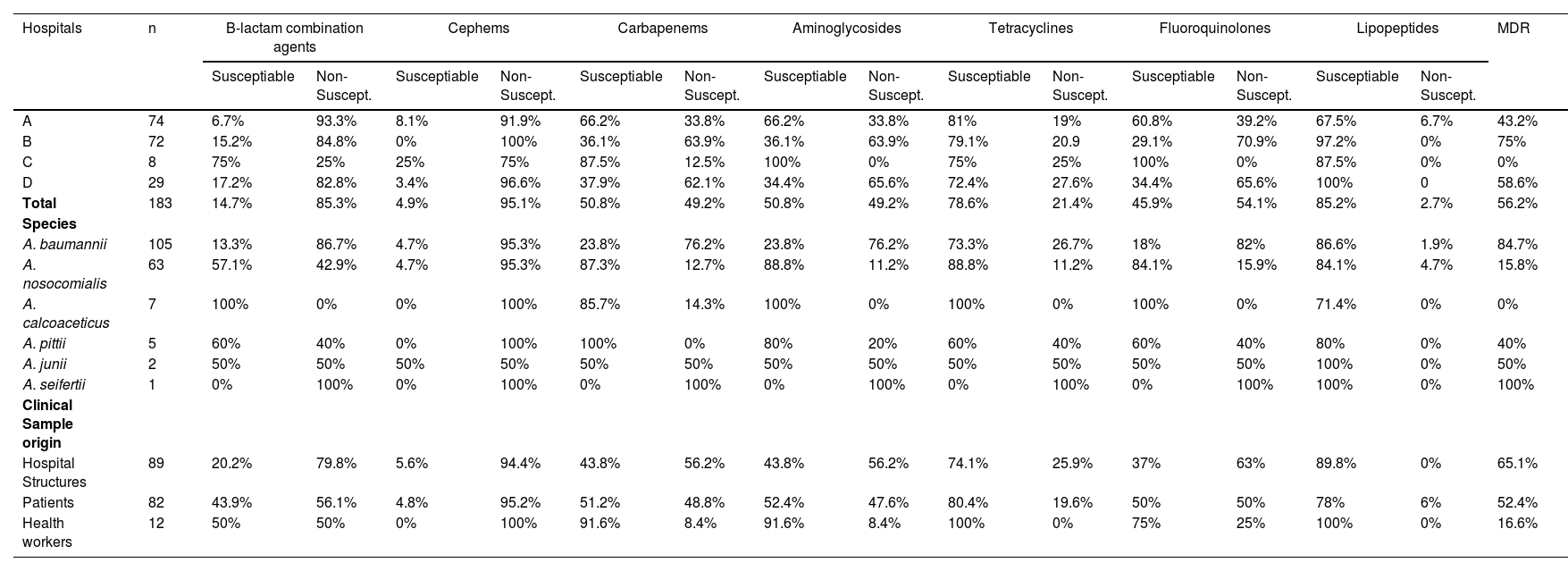
Based on the results obtained (Table 2), Hospital A had a lower percentage of MDR Acinetobacter spp. (43.2%, 32/74) and hospital B there was a higher prevalence (75%, 54/72). By evaluating only Hospitals A and B, it can be inferred that strains isolated from Hospital B were four times more likely to present a MDR profile than those isolated from Hospital A (Odds Ratio = 3.9375, p = 0.0002).
Resistance classification and percentage values of sensibility tests with Acinetobacter spp. isolated.
Notes: MDR, Multidrug-Resistant.
Similarly, samples of Acinetobacter spp. from hospitals structures showed the highest levels of MDR at 65.2% (58/89), followed by those from patients and healthcare professionals at 52.4% (43/82) and 16.6% (2/12), respectively. A. baumannii showed a significantly higher rate of MDR than the other species (p < 0.001), representing 90.3% (93/105) of samples.
DiscussionThe genus Acinetobacter has emerged as a worldwide public health concern because of its ability to cause HAIs and resist currently available antimicrobial treatments. In the present study, we aimed to identify Acinetobacter spp. and the resistance profiles of samples collected from patients, health professionals, and hospital environmental sources in the ICUs of different public reference hospitals in the city of Porto Velho, the capital of Rondônia, Northern region of Brazil.
We found that A. baumanni and A. nosocomialis were the most prevalent species. Other species of clinical importance have also been isolated, most of which belong to the Acinetobacter baumannii-calcoaceticus complex. A. calcoaceticus has already been described in severe cases of human infections such as pneumonia.18,19A. nosocomialis and A. pittii are clinically important, as they are often reported to be MDR species and cause serious infections in healthcare facilities.20,21
The highest isolation rate of Acinetobacter spp. was from hospital environmental samples, with A. baumannii and A. nosocomialis being the most frequently isolated species and isolated mainly from beds and mechanical ventilation devices. Several studies have demonstrated the role of environmental surfaces near patients and the occurrence of A. baumannii XDR and MDR outbreaks in ICUs.22Acinetobacter spp. have a strong ability to form biofilms, resist desiccation and sterilization, and thus persist in hospital environments.23,24 Furthermore, isolation from a ventilation device is directly related to the reported high prevalence of Acinetobacter spp. that cause ventilator-associated pneumonia.25
In the current study, it was found that the highest number of Acinetobacter spp. from patients was from the oral cavity, which is consistent with previous studies that have shown that the oral cavity can act as a reservoir for pathogens causing severe lung disease.19,26A. baumannii was also observed in tracheostomy samples. Other studies have shown that infections at these surgical sites are the leading causes of morbidity and mortality in the ICU.27,28
Contrary to data reported in the literature, Acinetobacter spp. were identified at low frequencies in samples from healthcare workers. This is a positive result, because approximately 80% of HAIs are currently associated with hand transmission.29
High rates of bacteria not susceptible to combinatorial agents to β-lactams, cephalosporins, and carbapenems were observed. The MDR rates were >50%, with. A. baumannii being the species with the highest levels of resistance and MDR. Notably, A. baumannii has intrinsic resistance to several antimicrobials of the ꞵ-lactam class, justifying the high resistance rate to these antimicrobials in the present study.
In addition to intrinsic resistance, A. baumannii has a high capacity to develop resistance to virtually all classes of agents used in clinical practice, showing high rates of resistance to broad-spectrum β-lactams such as third generation cephalosporins and carbapenems. Resistance to carbapenems requires increased attention as they are one of the last therapeutic options for defense against gram-negative bacteria.12
The present study reported that 76.2% of A. baumannii samples were resistant to carbapenems, and >90% were classified as MDR. These results are similar to those of other Brazilian studies that demonstrated carbapenem resistance rates of 74%–100%.13,30–32 Thus, knowing the rates of CRAB is extremely relevant because these isolates are associated with high survivability and dissemination in the hospital environment when they colonize medical equipment and the hands of health professionals, as in the case of the present study, which is a propitious factor in the emergence of outbreaks.33–35
Currently, therapeutic options for infections caused by CRAB isolates are limited. We observed a higher sensitivity of A. baumannii isolates to polymyxin B (97.9%), followed by antibiotics from the tetracycline (83.8%) and aminoglycoside (54%) classes. Polymyxins have been adopted as the antimicrobials of last resort to combat carbapenem-resistant strains and can be used alone or in combination with other antibiotics. Despite their high levels of susceptibility, polymyxins have the disadvantage of being neurotoxic and nephrotoxic. The emergence of resistant clinical isolates in other countries and Brazil is a concern, highlighting the need to monitor the susceptibility level to this drug.36,37
The tested tetracyclines also showed high sensitivity (> 80%). However, their use is not recommended, because Acinetobacter spp. are intrinsically resistant to tetracyclines and doxycyclines.38 In addition, we also tested ampicillin-sulbactam, which is within the CLSI recommendations, showing more than 50% sensitivity in our isolates. However, high error rates have been reported when using the disk-diffusion methodology to detect resistance to ampicillin-sulbactam.39
Antibiotics in the aminoglycoside class showed the third highest susceptibility rate. In this study, we found that gentamicin, among the aminoglycosides, was more effective against Acinetobacter spp. isolates with lower levels of resistance than in other Brazilian studies.31,32 Although they present with nephrotoxicity and ototoxicity, these antibiotics are also used as alternatives against MDR microorganisms but have to be associated with another antimicrobial, usually a cephalosporin, fluoroquinolone, or carbapenem.40
In Brazil, Technical Note n° 347/2021 ‒ CGLAB/DAEVS/SVS/MS presents guidelines for selecting antimicrobials to be tested and reports on antimicrobial sensitivity testing against carbapenem-resistant isolates of Acinetobacter spp. Based on this document, we highlighted the importance of creating institutional protocols with therapeutic steps, considering the site of infection, identifying the microorganism, and evaluating the sensitivity of the isolate.41
In conclusion, we report here for the first time the identification of Acinetobacter spp. from clinical samples of patients, health professionals, and hospital environments of ICUs of the four reference hospitals in the municipality of Porto Velho, Brazilian Amazon. Furthermore, we show that it is possible to establish the resistance profile of the eight identified species, with A. baumannii being the most predominant and associated with high rates of resistance and MDR. The MDR observed in environmental samples from ICUs highlights the potential for colonization and permanence at these sites, making it a possible reservoir for future outbreaks caused by these pathogens. Finally, we suggest that the data presented here can be used to guide and strengthen the control of MDR infections caused by Acinetobacter spp. in ICUs, as well as improve the current protocols and guidelines by providing information from a traditionally unassisted region of Brazil.
Authors’ contributionsMarcos Eduardo Passos da Silva: Conducted the experiments and manuscript writing.
Maicon Aleandro da Silva Gomes: Conducted the experiments and manuscript writing.
Renata Santos Rodrigues: Data analyses and manuscript review.
Núcia Cristiane da Silva Lima: Conducted the experiments.
Anjo Gabriel Carvalho: Data analyses and manuscript review.
Roger Lafontaine Mesquita Taborda: Analyses of the epidemiology informations.
Najla Benevides Matos: Conceined and designed of the study and review the results and the manuscript.
FundingThis Study was financial support by Fundação Oswaldo Cruz – FIOCRUZ RÔNDONIA; Instituto Nacional de Epidemiologia na Amazônia Ocidental (INCT EpiAMO), do Centro de Pesquisa em Medicina Tropical – CEPEM/RO; Programa de Pesquisa para o SUS (PPSUS); Fundação de Amparo à Pesquisa do Estado de Rondônia
We are very grateful to Andrea Barbieri de Barros, Infectologist at Hospital de Base Ary Pinheiro of Porto Velho City, for her intense support; To the patients who participated in the study and to the Fundação Conrado Wessel in memorian of Erney Plessmann de Camargo.