Symptomatic forms of toxoplasmosis are a serious public health problem and occur in around 10–20% of the infected people. Aiming to improve the molecular diagnosis of symptomatic toxoplasmosis in Brazilian patients, this study evaluated the performance of real time PCR testing two primer sets (B1 and REP-529) in detecting Toxoplasma gondii DNA. The methodology was assayed in 807 clinical samples with known clinical diagnosis, ELISA, and conventional PCR results in a 9-year period. All samples were from patients with clinical suspicion of several features of toxoplasmosis. According to the minimum detection limit curve (in CT), REP-529 had greater sensitivity to detect T. gondii DNA than B1. Both primer sets were retrospectively evaluated using 515 DNA from different clinical samples. The 122 patients without toxoplasmosis provided high specificity (REP-529, 99.2% and B1, 100%). From the 393 samples with positive ELISA, 146 had clinical diagnosis of toxoplasmosis and positive conventional PCR. REP-529 and B1 sensitivities were 95.9% and 83.6%, respectively. Comparison of REP-529 and B1 performances was further analyzed prospectively in 292 samples. Thus, from a total of 807 DNA analyzed, 217 (26.89%) had positive PCR with, at least one primer set and symptomatic toxoplasmosis confirmed by clinical diagnosis. REP-529 was positive in 97.23%, whereas B1 amplified only 78.80%. After comparing several samples in a Brazilian referral laboratory, this study concluded that REP-529 primer set had better performance than B1 one. These observations were based after using cases with defined clinical diagnosis, ELISA, and conventional PCR.
The infection by Toxoplasma gondii in humans is generally asymptomatic. During the chronic phase, the parasites persist encysted in the brain and muscle in the majority of the cases.1–3 However, symptomatic forms may occur in around 10–20% of the infected people. Thus, toxoplasmosis could be a serious public health problem, as it may lead to more severe symptoms.4–7
Infections occurring during pregnancy can result in severe neonatal impairment in the fetus. In the ocular form, lesions result from congenital or after birth-acquired infections and normally are necrotic. These lesions may destroy the architecture of the neural retina and sometimes involve the choroid (retinochoroiditis).8–13
The reactivation of latent infection as result of an immunodeficiency such as severely immunosuppressed HIV-infected patients in absence of highly active antiretroviral therapy and prophylaxis results more frequently in cerebral toxoplasmosis. In rare cases, disseminated toxoplasmosis can occur, which involves at least two organs, in failure of an effective Th1 immune response.5,6,14–18
The laboratory diagnosis is based on serological and molecular tests (PCR). In ocular form, the diagnosis is typically clinical but the laboratory tests normally provide confirmation.13,19 Serological diagnosis regularly monitors seronegative pregnant women and seroconversion of children under congenital toxoplasmosis investigation.20 In case of seroconversion, detection of T. gondii DNA by PCR in amniotic fluid (AF) confirms congenital toxoplasmosis.21–24 After birth, congenital diagnosis can be made in cerebrospinal fluid (CSF) or blood (BL) samples from newborns. In cases of suspicion of cerebral or disseminated toxoplasmosis, the treatment is usually initiated based on clinical, radiological, and serological features.6,15 PCR has been shown to be an important diagnostic tool since it is used in all forms of symptomatic toxoplasmosis.25–29 Such diagnosis leads to rapid and specific treatment, thus avoiding, or reducing serious damage caused by T. gondii.
Real-time quantitative PCR (qPCR) has been used for molecular diagnosis and studies demonstrated qPCR applicability in toxoplasmosis diagnosis using different targets.24–26,28,30–34
Aiming to improve molecular diagnosis of symptomatic toxoplasmosis in Brazilian patients, this study evaluated the performance of qPCR in detecting T. gondii DNA using two primer sets for routine diagnosis. These markers are used in laboratories in European countries for routine diagnosis.23,29,35
Material and methodsPatients and clinical samplesThis retrospective and prospective study evaluated clinical samples from 807 patients. Instituto Adolfo Lutz (São Paulo, Brazil) received these samples from January 2008 through November 2016 for molecular diagnosis of toxoplasmosis. Clinical samples originated from patients with known final clinical diagnosis were chosen. The clinical samples analyzed during this period were as follows: 173 (2008–2010), 204 (2011–2013), 138 (2014–2015), and 292 (2016). These clinical samples sent to the laboratory included CSF (302), BL with EDTA (439), AF (47), and formalin-fixed paraffin-embedded (FFPE) tissues sectioned in 4-μm-thick (19) (AU). For serological tests, BL samples were collected in tubes with no anticoagulants. Clinical samples for PCR and serology were sent to the laboratory within 48h after collection, and immediately processed. In the retrospective part of this study, the routine diagnosis of 515 clinical samples collected until 2015 included conventional PCR (cPCR) and enzyme-linked immunosorbent assay (ELISA). In the prospective study, the 292 clinical samples sent to the laboratory in 2016 were analyzed by qPCR using the primers sets REP-529 and B1.
The samples evaluated in this study were from symptomatic patients with the following clinical diagnosis of toxoplasmosis: cerebral (404 HIV-patients), ocular (193 with ocular alterations), congenital infection by transplacental route (125 newborns), gestational infection (66 pregnant women), and disseminated (19 brain autopsies from deceased HIV-patients).
HIV-infected patients with suspicion of cerebral toxoplasmosis presented progressive neurological deficits and contrast-enhancing mass lesion(s) on computed tomography or magnetic resonance imaging. These cases were included in “definition for cerebral toxoplasmosis” previously described.6,15 Those who died from severe disseminated toxoplasmosis had positive serology and immunohistochemistry for toxoplasmosis of at least two organs. Both group of patients were admitted and treated at the Instituto de Infectologia Emilio Ribas by Dr Jose E. Vidal's group. Patients with suspicion of ocular toxoplasmosis had ocular alterations, such as toxoplasmic retinochoroiditis scars or retinal exudative lesions. They were admitted and treated at Ambulatorio de Oftalmologia, by Dr Fábio B. Frederico's group. Pregnant women with suspicion of acute toxoplasmosis infection were admitted and treated at the High Risk Antenatal Care and Fetal Medicine Service. After enrolling in a high-risk pregnancy clinic, pregnant women were routinely screened for TORSCH (Toxoplasmosis, Rubella, Syphilis, Cytomegalovirus, Hepatitis and HIV).36,37 Those clinically, epidemiologically suspected of toxoplasmosis and having positive IgM or low IgG avidity for anti-T. gondii antibodies underwent amniocentesis for PCR testing in amniotic fluid. Before collection pregnant women were informed of the procedure and required to sign informed consent forms. The newborns of these mothers with suspected or confirmed fetal infection were admitted and treated at the High Risk Pediatric Service and Fetal Medicine Service. Mothers and newborns were treated by Dr Lígia CJF Spegiorin's group.
T. gondii antigen and serological diagnosisT. gondii lysate antigen and ELISA were performed exactly as described before.19,38,39
DNA purificationBL samples (5mL) were prepared as described before.28 Plasma samples were separated by centrifugation and used in serological diagnosis. Pellets were mixed with three times the volume of a buffer containing 150-mM ammonium chlorate, 1-mM potassium bicarbonate, 0.1-mM EDTA, pH 7.3, incubated for 15min at room temperature under mild shaking and centrifuged (2500g/10min). AF samples (10mL) were centrifuged (2500g/10min) and the supernatant was discarded. Blood pellets, containing only nuclei cells, AF packed cells and crushed tachyzoites (for control and standard curves) were digested for 10min at 56°C with proteinase K (20μg) in 200μL of AL buffer (Qiagen). AU fragments (5 sections of 4-μm-thick FFPE) were dissolved in xylene (1mL), incubated for 30sec, centrifuged twice (8000g/1min) and supernatants were removed. Pellets were mixed with 1mL ethanol, centrifuged (8000g/1min) and incubated for 10min, at room temperature for complete xylene evaporation.40
DNA molecules were extracted from BL, AF, and tachyzoites by QIAamp DNA Mini Kit (Qiagen); and from AU, by QIAamp DNA FFPE Tissue kit (Qiagen). The protocols were followed according to the manufacturer's instructions in a Robotic workstation for automated purification of DNA (QIAcube, Qiagen). DNA molecules from CSF samples (3mL) were extracted as described before.41 DNA concentrations and purity were determined by the ratio of O.D. at 260 and 280nm in NanoDrop ND1000. For use in PCR, samples with high DNA concentrations (as some BL and AU) were diluted with ultra-pure water until concentration 100ng/μL.
cPCR target for toxoplasmosis and internal controlT. gondii was identified by cPCR using the primer pair B22-B23,31 designed to amplify a 115-bp amplicon from the B1 gene as target. The reactions were run following the same conditions described elsewhere.42 Absence of PCR inhibitors was verified using a housekeeping gene that amplified a 140bp fragment of the human β-globulin gene.43 Each amplification run contained two negative controls (ultra-pure water and negative DNA for toxoplasmosis) and one positive (DNA extracted from tachyzoites). After thermal cycles, PCR products were electrophoresed in 2% agarose gel and stained with ethidium bromide and visualized under UV illumination.
qPCR target for toxoplasmosis and internal controlThe performance of two different primer sets for qPCR was analyzed. The first (B1) amplified a 71-bp fragment of the B1 gene as template,29,35 which has 35 copies in the genome and is conserved in different parasite strains.31 The design included the forward (5′-GAAAGCCATGAGGCACTCCA-3′) and reverse (5′-TTCACCCGGACCGTTTAGC-3′) primers; and a hybridization probe (5′-CGGGCGAGTAGCACCTGAGGAGATACA-3′) labeled with FAM (6-carboxyfluorescein) and BHQ1 (Black Hole Quencher 1) at the 5′ and 3′ ends, respectively.
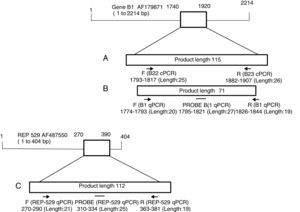
The second (REP-529) amplified a 112-bp of the highly repetitive sequence REP-529 (Genbank AF487550),23,29 which has 200–300copies in T. gondii genome.30 The design included the forward (5′-AGAGACACCGGAATGCGATCT-3′) and reverse (5′-TTCGTCCAAGCCTCCGACT-3′) primers; and the hybridization probe (5′-TCGTGGTGATGGCGGAGAGAATTGA-3′) labeled with FAM and BHQ1. The design and location in the gene of each primer pair is described in Fig. 1. Both molecular marker sequences were previously standardized in our laboratory using serial dilutions of DNA from tachyzoites (RH strain) as template. To confirm the absence of PCR inhibitors, a primer set to a Eukaryotic 18S rRNA gene (GenBank accession code X03205.1), purchased from Applied Biosystems was used as housekeeping gene. The reactions were performed in final volume of 20μL. DNA samples (3μL of DNA until 100ng/μL), DNA control (5ng/μL) or DNA for standard curve (3μL for each point) were added to reaction mixture containing 10μL of 2X TaqMan Universal PCR Master Mix. Next, 1.25μL of “Assay Mix” (18μM of forward and reverse primers and 5μM of the hydrolysis probe). Amplifications were performed in an Applied Biosystems 7500 Real-time PCR System using the following thermal profile: 2min, 50°C, and 95°C for 10min. Next, 40 cycles were performed at 95°C for 15sec and 60° C for 1min.
Design and genes location of primer sets used in this study. (A) cPCR (B22-B23), (B) qPCR B1, and (C) qPCR REP-529. The analyses were done in https://www.ncbi.nlm.nih.gov/tools/primer-blast/primertool.cgi?ctg_time=1486921082&job_key=1N4LxE9DQutl1VjQVbB84i-rbdACuHbNAw&CheckStatus=Check for B22/B23; https://www.ncbi.nlm.nih.gov/tools/primer-blast/primertool.cgi?ctg_time=1486924834&job_key=l51I5ugp5YHCu3W-eN5RjALFQL4v1lujLg for (B1), and https://www.ncbi.nlm.nih.gov/tools/primer-blast/primertool.cgi?ctg_time=1487009304&job_key=ycMWuJUKmKK_mAidBf0sr3_mPZ1S9SaAUw&CheckStatus=Check for REP-529. The schemes were based on Lin et al.46
Two T. gondii standard curves using REP-529 and B1 primer sets were constructed in nine concentrations of DNA obtained from 1×107 tachyzoites (in triplicate). Initial concentration (1×107 tachyzoites) was 35ng/μL and serial dilutions ranged up to 10−1 tachyzoites (3.5fg). The cycle threshold values (CT) were plotted as mean (triplicate) against the standard curve values to determine the detection limit of both primer sets. Parasite concentrations were determined after the calculation of the linear regression equation (y=ax+b), where y=CT; a=curve slope (slope); x=parasite number; and b=where the curve intersects y-axis (y intercept).44
Quality assuranceIn each PCR run, a blank control was used consisting of DNA-free water plus the PCR mix. Separate rooms were used for (i) DNA extraction; (ii) PCR mix and primer preparation; (iii) adding DNA from clinical samples and positive control; and (iv) post-PCR agarose gel electrophoresis analysis in case of the cPCR. DNA samples were assayed in triplicate and, at least twice to determine reproducibility. The quality of DNA samples stocked for nine years and retrospectively re-analyzed with qPCR was confirmed by the positivity using the 18S primer set.
Data analysisThe discordant results in qPCR were re-analyzed, using the same protocols, at least twice. Sensitivities and specificities of both primer sets in clinical samples were determined considering the true diagnosis (clinical and laboratory diagnoses) and were calculated as: (i) percent of sensitivity=ratio of true positives/true positives+false negatives×100; (ii) percent of specificity=ratio of true negatives/(true negatives+false positives)×100. Reproducibility calculations were done exactly as described before.45 The percentages of concordant indices were calculated as the following ratio: (number of concordant results)/(number total of samples)×100. Linear regressions were constructed from the standard curves for REP-529 and B1 and CT mean comparison of both primer pairs. Both curves were statistically analyzed using an unequal-variance t-test based on a critical value of p≤0.05. All calculations were run in GraphPad Prism 6.0 software.
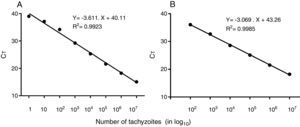
ResultsREP-529 and B1 tested in T. gondii tachyzoitesThe first step was to evaluate the reportable range of the reaction that was calculated using DNA extract from tachyzoites (RH strain) using the REP-529 (Fig. 2A) and B1 (Fig. 2B). The resulting standard curve showed the detection limit of each curve. R2=0.9923 for REP-529 and 0.9985 for B1 showed high linearity among the variables.46 The minimum detection limit (in CT) was 39.28 (corresponding to one parasite) for REP-529, higher than the 37.18 (corresponding to 100 parasites) for B1.
Standard curve of T. gondii, tachyzoites using REP-529 (A) and B1 (B) primer sets, respectively using the hydrolysis probe FAM dye-labeled. Results are shown as mean cycle threshold (CT) obtained from triplicate of each DNA concentration. Standard curve analysis was done in 10-fold serial dilutions of DNA extracted from tachyzoites, at initial concentration of 35ng/μL (1×107 tachyzoites). For REP-529, R2=0.9923 and B1, R2=0.9985.
The first 515 DNA samples (collected until 2015) had results of clinical diagnosis, ELISA and cPCR (B22-B23). These DNA samples were used to test REP-529 and B1 in qPCR, and results are shown in Table 1. DNA samples of 122 patients had negative serological, molecular, and clinical diagnosis (without toxoplasmosis). REP-529 and B1 provided specificities of 99.2% and 100%, respectively. All samples were negative when considering results of at least one primer set.
Summary of retrospective of qPCR analysis employing REP-529 and B1 primer sets in 515 DNA samples with results in clinical diagnosis, ELISA, and cPCR (B22-B23).
| Clinical samples | Results | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Serological diagnosis (ELISA) | n | cPCR (B22-B23) | qPCR (REP-529) | qPCR (B1) | |||||
| Negative | Positive | Negative | Positive | % | Negative | Positive | % | ||
| Negative | 122 | 122 | 0 | 121 | 1 | 99.2a | 122 | 0 | 100a |
| Positive | 393 | 247 | 146b | 253 | 140 | 95.9b | 281 | 122 | 83.6 |
| Total | 515 | 369 | 146 | 374 | 141 | – | 403 | 122 | – |
Table 1 also shows PCR results of 393 clinical samples with positive ELISA. Positive IgG antibodies anti-T. gondii are not necessarily indicative of a symptomatic infection, whereas detection of tachyzoite DNA in biological fluids as cerebral, ocular, congenital or disseminating forms can characterize a symptomatic infection.19,40,42,47,48 Thus, sensitivities of REP-529 and B1 were 95.9% and 83.6%, respectively were calculated considering cPCR and clinical diagnosis (146 samples, which included 94 patients with cerebral toxoplasmosis, 10 newborns with congenital infection, 21 patients with ocular toxoplasmosis, six pregnant women with toxoplasmosis, and 15 postmortem patients with severe disseminated toxoplasmosis) (Table 2). REP-529 amplified 140/146 samples with average CT of 31.71±0.36. B1 amplified 122/146, with average CT of 32.12±0.38.
Clinical and PCR results of the 807 clinical samples analyzed.
| Toxoplasmosis (clinical form) | Total number of clinical samples analyzed | Positive samples clinical, molecular, and serological diagnosis | % Positivity (both studies) | ||
|---|---|---|---|---|---|
| Retrospective study (total – 515 samples) | Prospective study (total – 292) | Both studies (total – 807 samples) | |||
| Cerebral | 404 | 94 | 31 | 125 | 30.94 |
| Congenital | 125 | 10 | 6 | 16 | 12.80 |
| Gestational | 66 | 6 | 6 | 12 | 18.18 |
| Ocular | 193 | 21 | 25 | 46 | 23.83 |
| Disseminated (postmortem) | 19 | 15 | 3 | 18 | 94.74 |
| Total | 807 | 146 | 71 | 217 | 26.89 |
In the prospective study (2016), a total of 292 clinical samples were analyzed by REP-529 and B1. As shown in Table 2, among 807 clinical samples, 217 (26.89%) had positive PCR, at least one primer set and a confirmed clinical diagnosis. These included: (i) 30.94% (125/404) patients with cerebral toxoplasmosis; (ii) 12.80% (16/125) newborns with congenital alterations; (iii) 18.18% (12/66) of pregnant women with acute toxoplasmosis with T. gondii DNA in AF or BL; (iv) 23.83% (46/193) patients with ocular alterations and T. gondii DNA in BL; and (v) 94.74% (18/19) postmortem patients with severe disseminated toxoplasmosis.
Table 3 describes the performance of primer sets and clinical characteristics of 217 positive samples. REP-529 amplified T. gondii DNA in 97.23% (211/217) of positive samples, and B1 were positive only in 78.80% (171/217) of them.
Characteristics of clinical samples, sensitivities of REP-529 and B1 considering the 217 positive samples.
| Clinical form (n) | Sample | Totala | REP-529 | B1 | ||
|---|---|---|---|---|---|---|
| Positive | % | Positive | % | |||
| Cerebral (125) | CSF | 49 | 49 | 100 | 41 | 83.67 |
| BL | 76 | 72 | 94.74 | 62 | 81.58 | |
| Congenital (16) | CSF | 3 | 3 | 100 | 2 | 66.67 |
| BL | 13 | 13 | 100 | 9 | 69.23 | |
| Gestational (12) | AF | 8 | 8 | 100 | 5 | 62.50 |
| BL | 4 | 4 | 100 | 3 | 75.00 | |
| Ocular (46) | BL | 46 | 44 | 95.65 | 36 | 78.26 |
| Disseminated (18) | AU | 18 | 18 | 100 | 13 | 72.22 |
| Total | 217 | 211 | 97.23 | 171 | 78.80 | |
Although average CT of REP-529 is lower than for B1, the CT with both primer sets were not statistically different in positive samples (Table 4).
Comparison between mean CT values obtained with REP-529 and B1 qPCR considering the 217 positive samples.
| Clinical form (n) | Sample | Totala | Mean±SEM (n) CT | p valueb | ||
|---|---|---|---|---|---|---|
| REP-52 | B1 | ΔCT | t test | |||
| Cerebral (125) | CSF | 49 | 32.86±0.43 (49) | 33.71±0.40 (41) | 0.85 | 0.2420 (ns) |
| BL | 76 | 31.08±0.54 (72) | 31.92±0.51 (21) | 0.84 | 0.2830 (ns) | |
| Congenital (16) | CSF | 3 | 35.61±0.22 (3) | 36.37±1.67 (2) | 1.68 | 0.2628 (ns)c |
| BL | 13 | 31.06±1.06 (13) | 32.99±1.13 (9) | 1.93 | 0.6757 (ns) | |
| Gestational (12) | AF | 8 | 34.69±0.63 (8) | 36.13±0.56 (3) | 1.44 | 0.0554 (ns) |
| BL | 4 | 35.02±0.58 (4) | 36.36±0.21 (2) | 1.34 | 0.0192 (ns) | |
| Ocular (46) | BL | 46 | 32.07±0.62 (44) | 33.22±0.66 (24) | 1.15 | 0.0225 (ns) |
| Disseminated (18) | AU | 18 | 30.92±0.96 (18) | 31.27±1.48 (10) | 0.35 | 0.2172 (ns) |
The concordance index among the primer sets was calculated based on the positive results. The total number of samples was based on the results of 515 clinical samples with positive (146) and negative (369) results in cPCR (B22-B23) until 2015, and 807 clinical samples with positive (217) and negative (590) results in qPCR (REP529 and B1) (2008–2016). As shown in Table 5, concordance of results among the three primer sets was 83.56%. Concordance between B22-B23 and REP-529 was 95.89%; between B22-B23 and B1 was 83.56%; and between REP-529 and B1, 83.87%.
Concordance index between three markers for detecting T. gondii in clinical samples.
| Period of the study | Primer sets | Clinical samples with symptomatic toxoplasmosis | PCR results | ||
|---|---|---|---|---|---|
| Concordanta | Discordantb | Concordance indexc (%) | |||
| B22-B23/REP-529/B1 | 146 | 122 | 24 | (83.56) | |
| 2008–2015 | B22-B23/REP-529 | 146 | 140 | 06 | (95.89) |
| B22-B23/B1 | 146 | 122 | 24 | (83.56) | |
| 2008–2016 | REP-529/B1 | 217 | 182 | 35 | (83.87) |
Percents of concordant index were calculated as the ratio: (number of concordant results)/(number total of samples)×100.
Number total of samples was based on results of 515 clinical samples with positive (146) and negative (369) results in cPCR (B22-B23) until 2015; and 807 clinical samples with positive (217) and negative (590) results in qPCR (REP529 and B1) (2008–2016).
Until 2015, molecular diagnosis in our laboratory included cPCR using primer set B22-B23, which amplified a 115-base-pair sequence of B1 gene.31 B22-B23 amplifies and detects DNA of a single organism directly from a crude cell lysate or 10 parasites in the presence of 100,000 human leukocytes.31 High sensitivity and specificity of this primer set were previously reported in CSF and BL samples collected from AIDS patients and from those with ocular toxoplasmosis in our setting.19,41,42 Thus, to include qPCR in molecular diagnosis, we previously evaluated the performance of qPCR to detect T. gondii DNA testing two primer sets.23,29,35
The first experiments were aimed to determine the minimum detection limit curve (in CT) of each primer set. REP-529 was more sensitive to detect T. gondii DNA than B1, which were 39.28 (corresponding to one parasite) and 37.18 (corresponding to 100 parasites), respectively. Similar results were shown in other study33 and they could be attributed at the number of repeated copies in T. gondii genome (B1 gene – 35 copies and 529bp sequence 200–300 copies).33 In fact, the REP-529 sequence is more abundant in number of repetitions than the B1.30 Despite these differences of sensitivity, good reproducibility (100%) was shown with both primer sets, since similar results were presented in the replicas (in triplicate) and in different reactions (same sample analyzed on different days).
Next, REP-529 and B1 were retrospectively evaluated using 515 DNA extracted from different clinical samples collected from Brazilian patients with known clinical and laboratorial diagnoses. The sensitivity and specificity of diagnostic tests were assessed on optimal working conditions. To improve the PCR sensitivity, the clinical samples were processed rapidly within 48h of collection to prevent Taq polymerase inhibition.42,49 Considering the variation of the parasite DNA concentration, all samples used in this study were quantified after extraction (since concentrations up to 100ng/μL can inhibit the reaction) and were checked for the DNA inhibitors by amplifying a human housekeeping gene. This main quality control prevented negative results due to low DNA quality.50 The quality of DNA samples stocked for up to nine years and retrospectively re-analyzed with qPCR was confirmed by positivity using the 18S primer set. These results were essential to verify if long-term storage at −20°C after initial diagnosis had not altered DNA. In addition, all discordant results between the three markers were repeated (in triplicate) at least twice.
Only one DNA (0.8%) sample had positive qPCR in REP-529 from the 122 patients without toxoplasmosis (they had negative ELISA, cPCR, and clinical diagnosis). Similar results have been shown in other studies.28,51 Thus, both primer sets provided high specificities (99.2% and 100%).
From the 393 clinical samples with positive ELISA, 146 had positive cPCR and these patients had symptomatic infection, according to the clinical diagnosis. Considering both cPCR and clinical diagnosis REP-529 and B1 sensitivities were 95.9% and 83.6%, respectively. The means of CT for REP-529 and B1 were 31.71±0.36 and 32.12±0.38, respectively. Although DNA samples stocked up to nine years were checked using two human housekeeping genes (β-globulin and 18S primer sets), six (2.8%) were false negative samples determined by REP-529 and 24 (16.4%) by B1 in the first part of the study (retrospective). These results could be attributed to: (i) degradation of T. gondii DNA by the long-term storage at −20°C; (ii) absence of such sequences in some of the biological samples as mentioned by others51; and (iii) human DNA could inhibit PCR when targeting the B1 gene, which occurs in 35 copies in T. gondii genome or in 529bp sequence, which occurs in more copies (200–300).30,33 Despite that, these results confirm that REP-529 was considerably better than B1 in detecting T. gondii DNA. These data have been confirmed by other studies.29,32,33,52
When only the qPCR for both primer sets were tested in molecular diagnosis, the reactions were done using DNA extracted recently. Comparison of REP-529 and B1 performances was also prospectively analyzed in 292 clinical samples collected in 2016 (Table 2). Thus, a total of 807 DNA samples were analyzed retrospectively and prospectively. Of them, 217 (26.9%) had positive PCR, at least one primer set and clinical diagnosis confirmed as symptomatic toxoplasmosis. REP-529 amplified T. gondii DNA in 211 (97.23%) clinical samples, whereas B1 amplified only 78.80% of them. Positive results determined by REP-529 in 18 AU brain samples were also confirmed by parasite visualization by immunohistochemistry in brain tissues. However, five of them had negative results when the B1 was used. Although these analyses were done in Brazilian clinical samples, they confirm findings of other studies conducted in European clinical samples aiming to compare sensitivities of both DNA regions, which showed that those from repeat regions were more sensitive than those from the B1 gene.26,29,33,52
The concordance index between three primer sets (B22-B23/REP-529/B1) varied from 83.56% to 95.89%. Although the B22-B23 and REP-529 genes amplify different T. gondii genic regions, both had almost the same power in detecting T. gondii. On the other hand, the concordance indices between B1 and REP-529 or B22-B23 were 83.87 and 83.56 respectively. The combination of B1 with other primer sets did not increase PCR sensitivity, since no sample were positive for B1 and negative for REP-529. Similar the results have been previous reported.52 Despite B1 and B22-B23 amplify the same B1 gene region (Fig. 1), they presented different sensitivities. Although T. gondii genotyping was not determined in the 217 patients with positive PCR, probably, different Brazilian genotypes could be circulating among these patients, as already showed by others.53,54 One small confirmation of these data was verified by the genotyping of 15 of the 18 AU brain samples used here. Among them, six genotypes were determined in our previous study.40 One of them was Toxo DB #11, previously identified in different Brazilian infected newborns and domestic animals.54 The other five genotypes identified were TgHuDis1, TgHuDis3 and TgHuDis5, TgHuDis2 and TgHuDis4.40 Although REP 529 has been regarded with a region with a large variation in its copy number in different T. gondii strains,52 these results can suggest that independent of the infecting strain, REP-529 can be satisfactorily used for molecular diagnosis of symptomatic toxoplasmosis.
In conclusion, this comparative study, which evaluated several samples in a Brazilian referral laboratory concluded that REP-529 had better performance than B1 one. These observations were concluded after using cases with defined clinical diagnosis, ELISA, and cPCR.
Ethics approval and consent to participateThe Ethics Committee of all involved Institutions approved the entire study, which was performed following the recommendations of the same Committee (CONEP-IAL/SES number: 815489).
Consent to publishAll authors revised the manuscript, approved the final version submitted, published, and agreed to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
FundingThis study was funded by grants from FAPESP (Fundação de Amparo à Pesquisa do Estado de Sao Paulo, Brazil) 2014/09496-1 to VLP-C, 2013/10050-5 to MP, 2013/15879-8 to FHAM, 2013/25650-8 to LCM, 2014/01706-7 to MNF; by CNPq (Conselho Nacional de Desenvolvimento Científico e Tecnológico) to VLP-C 301369/2015-1.
Authors’ contributionsVL Pereira-Chioccola, LM Camilo and CS Meira-Strejevitch designed the study and experiments; performed the data analysis, interpreted the data. VL Pereira-Chioccola wrote the manuscript. LM Camilo and R Gava performed the laboratorial experiments (DNA isolation, cPCR, qPCR, ELISA and data analysis). JE Vidal critically revised the manuscript and determined the clinical diagnosis for patients with cerebral and disseminated toxoplasmosis. CC Brandão de Mattos, LC Mattos, FB Frederico, RC Siqueira, M Previato, AP Barbosa, FHA Murata, MN Ferreira, LCJF Spegiorin, DMU Barbosa, FS Gonçalves, CM Dias, MW Catelan D. Cavallini performed the inclusion of patients with ocular, congenital, and gestational toxoplasmosis, sample collection, develop the clinical diagnosis and evaluation in FAMERP.
Conflicts of interestThe authors declare no conflicts of interest.
Jim Hesson of Academic English Solutions proofread the paper (http://academicenglishsolutions.com/AES/home.html).