Leprosy, a disease neglected in many countries, is endemic in Brazil. With a wide diversity of fauna distributed in three biomes (Amazon Forest, Cerrado and Pantanal), the state of Mato Grosso (MT) in the Central-West Region has the highest prevalence of human cases: 7.75 per 10,000 inhabitants.1 Despite the scarcity of data in the literature on wild animals naturally infected with Mycobacterium leprae, the possibility of transmission to humans cannot be ruled out. Armadillos, red squirrels, and non-human primates are important natural reservoirs of M. leprae reported in the literature, becoming possible sources of bacillary dissemination making it difficult to interrupt the leprosy transmission chain.2 As data on natural infections are scarce, it is difficult to understand the role of wild animals in transmission of the disease. Therefore, we used PCR to detect the genetic material of M. leprae in nasal swabs of wild animals.
Nasal swabs were collected from 69 captive and free wild animals from the MT and Pantanal regions of Brazil, independent of clinical signs, and sent to the Laboratory of Microbiology and Molecular Biology, according to “Sistema de Autorização e Informação em Biodiversidade” (SISBIO), an authorization and information system for biodiversity (nos. 40617-1 and 42303). The samples were submitted for extraction of genetic material according to the phenol/chloroform method. PCR was performed according to Woods and Cole.3 The PCR product was purified using a GFX™ PCR DNA and Gel Band Purification kit (GE Healthcare, Piscataway, NJ, USA) and sequenced using an ABI-PRISM 3500 Genetic Analyzer (Life Technologies Corporation, USA). The sequences were deposited in GenBank and compared using the BLAST program (http://www.ncbi.nlm.nih.gov/blast/Blast.cgi). Of the 69 samples (Table 1), six (8.69%) wild-type free and captive animals tested positive for M. leprae by PCR, including one margay (Leopardus wiedii), two lowland tapirs (Tapirus terrestris), two capuchin monkeys (Sapajus apella), and one owl monkey (Aotus trivirgatus). The detection in four different species of wild animals shows the ability of this bacillus to be carried in different hosts. In addition, two animals were from the zoo, that could have acquired M. leprae due to close contact to humans or environmental contamination. However, in literature the mechanism of transmission is not yet fully understood.4
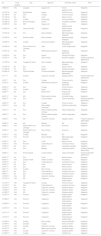
Epidemiological data and M. leprae PCR test of the animals identified in Mato Grosso – Brazil.
| ID | Free-ranging | City | Species | Scientific name | PCR |
|---|---|---|---|---|---|
| m962/16 | Yes | Jangada | Jaguarundi | Puma yagouaroundi | Negative |
| m1016/16 | Yes | Marcelândia | Jaguar | Panthera onca | Negative |
| m1102/16 | No | Zooa | Cougar | Puma concolor | Negative |
| m1122/16 | No | Zoo | Coati | Nasua nasua | Negative |
| m1162/16 | Yes | Cuiabá | Guinea pig | Cavia porcellus | Negative |
| m1226/16 | Yes | Barra do Bugres | Ocelot | Leopardus pardalis | Negative |
| m1285/16 | Yes | NAb | Jaguarundi | Puma yagouaroundi | Negative |
| m1294/16 | Yes | Várzea Grande | Capybara | Hydrochoerus hydrochaeris | Negative |
| m1335/16 | No | Zoo | Giant anteater | Myrmecophaga tridactyla | Negative |
| m1336/16 | Yes | Rosário Oeste | Giant anteater | Myrmecophaga tridactyla | Negative |
| m1364/16 | Yes | Cuiabá | Collared anteaters | Myrmecophaga tetradactyla | Negative |
| m1485/16 | Yes | Santo Antônio do Leverger | Otter | Lontra longicaudis | Negative |
| m1491/16 | Yes | NA | White-eared opossum | Didelphis albiventris | Negative |
| m1529/16 | No | Zoo | Agouti | Dasyprocta Aguti | Negative |
| m1787/17 | Yes | NA | Owl monkey | Aoutus trivirgatus | Positive Genbank MF975704 |
| m1790/16 | Yes | Tangará da Serra | Giant anteater | Myrmecophaga tridactyla | Negative |
| m1795/16 | No | Zoo | Coati | Nasua nasua | Negative |
| m1796/16 | No | Zoo | Agouti | Dasyprocta aguti | Negative |
| m1862/16 | Yes | Várzea Grande | Collared anteaters | Myrmecophaga tetradactyla | Negative |
| m11/17 | Yes | Cuiabá | Capuchin monkey | Sapajus apella | Positive Genbank MF975703 |
| m74/17 | No | Zoo | Cougar | Puma concolor | Negative |
| m153/17 | Yes | Cuiabá | White-eared opossum | Didelphis albiventris | Negative |
| m234/17 | No | Zoo | Cougar | Puma concolor | Negative |
| m235/17 | Yes | Cuiabá | Sagui | Callithrix sp. | Negative |
| m248/17 | No | Zoo | Maned wolf | Chrysocyon brachyurus | Negative |
| m261/17 | Yes | NA | Black owler monkey | Alouatta caraya | Negative |
| m305/17 | No | Zoo | Lowland tapirs | Tapirus terrestris | Positive Genbank MF975707 |
| m345/17 | No | Zoo | Cougar | Puma concolor | Negative |
| m379/17 | Yes | NA | Black-tufted marmoset | Callithrix penicillata | Negative |
| m514/17 | No | Zoo | White-cheeked spider monkey | Ateles marginatus | Negative |
| m520/17 | No | Zoo | White-cheeked spider monkey | Ateles marginatus | Negative |
| m530/17 | Yes | NA | Capuchin monkey | Sapajus apella | Positive Genbank MF818035 |
| m539/17 | Yes | NA | Monkey | NA | Negative |
| m542/17 | Yes | Santo Antônio do Leverger | Owl monkey | Aotus sp. | Negative |
| m543/17 | Yes | Santo Antônio do Leverger | Owl monkey | Aotus sp. | Negative |
| m705/17 | Yes | Cuiabá | Monkey | NA | Negative |
| m709/17 | Yes | Poconé | Giant anteater | Myrmecophaga tridactyla | Negative |
| m743/17 | No | Zoo | Coati | Nasua nasua | Negative |
| m748/17 | Yes | Poconé | Crab-eating fox | Cerdocyon thous | Negative |
| m765/17 | No | Zoo | Ocelot | Leopardus pardalis | Negative |
| m787/17 | No | Zoo | Margay | Leopardus weidii | Positive Genbank MF975706 |
| m809/17 | Yes | Rondonópolis | Lowland tapirs | Tapirus terrestris | Positive Genbank MF975705 |
| m874/17 | No | Zoo | Coati | Nasua nasua | Negative |
| m878/17 | Yes | Campo Verde | Howler monkey | Alouatta sp. | Negative |
| m879/17 | No | Zoo | Crab-eating fox | Cerdocyon thous | Negative |
| m742/17 | No | Zoo | Coati | Nasua nasua | Negative |
| m721/17 | No | Zoo | Coati | Nasua nasua | Negative |
| m871/17 | Yes | Cuiabá | Owl monkey | Aoutus azare | Negative |
| m897/17 | No | Zoo | Crab-eating fox | Cerdocyon thous | Negative |
| m881/17 | No | Zoo | Crab-eating fox | Cerdocyon thous | Negative |
| m1055/17 | Yes | NA | Capuchin monkey | Sapajus apella | Negative |
| m1070/17 | Yes | NA | Capybara | Hydrochoerus hydrochaeris | Negative |
| m1126/17 | Yes | Tangará da Serra | Cougar | Puma concolor | Negative |
| m1153/17 | No | Zoo | Crab-eating fox | Cerdocyon thous | Negative |
| m1247/17 | Yes | NA | Capuchin monkey | Sapajus apella | Negative |
| m1248/17 | Yes | NA | Capuchin monkey | Sapajus apella | Negative |
| m1249/17 | Yes | NA | Capuchin monkey | Sapajus apella | Negative |
| m1267/17 | Yes | Poconé | Capybara | Hydrochoerus hydrochaeris | Negative |
| m1268/17 | Yes | Poconé | Capybara | Hydrochoerus hydrochaeris | Negative |
| m1269/17 | Yes | Poconé | Capybara | Hydrochoerus hydrochaeris | Negative |
| m1270/17 | Yes | Poconé | Capybara | Hydrochoerus hydrochaeris | Negative |
| m1271/17 | Yes | Poconé | Capybara | Hydrochoerus hydrochaeris | Negative |
| m1272/17 | Yes | Poconé | Capybara | Hydrochoerus hydrochaeris | Negative |
| m1290/17 | Yes | Cuiabá | Owl monkey | Aoutus nigriceps | Negative |
| m1309/17 | Yes | Cáceres | Cougar | Puma concolor | Negative |
| m1313/17 | Yes | Cuiabá | Capuchin monkey | Sapajus apella | Negative |
| m1327/17 | Yes | NA | Black owler monkey | Alouatta caraya | Negative |
| m1338/17 | Yes | Cuiabá | Capuchin monkey | Sapajus apella | Negative |
| m1339/17 | Yes | Cuiabá | Sagui | Callithrix sp. | Negative |
Knowledge of the environment surrounding the infected humans or animals, and route of infection and mode of transmission are necessary to understand endemics in certain regions.4 Truman et al.5 described that isolates from human and armadillos are identical genetically. Thus, we suggest that the possible contact of animals of this study, which may be possible carriers of the bacillus, with other animals or with humans can disseminate the disease, the bacillus was detected in nasal swabs. Thus, we observe that the detection in wild animals may be associated with high prevalence and endemicity in the state of MT, which makes them important sources of infection. In addition, these data contribute to a better understanding of the epidemiology of leprosy.
DisclaimersThe opinions expressed by authors contributing to this journal do not necessarily reflect the opinions of the Centers for Disease Control and Prevention or the institutions with which the authors are affiliated.
Conflicts of interestThe authors declare no conflicts of interest.
The authors are grateful to CAPES for financial support through a scholarship.