Human parvovirus B19 is a well-known cause of severe conditions in patients with sickle cell disease, but the molecular mechanisms of the infection are insufficiently understood. The different clinical outcome of the acute parvovirus B19 infection in two pediatric patients with sickle cell disease has been examined. One of them developed life-threatening condition requiring emergency transfusions, while the other had asymptomatic infection, diagnosed occasionally. Both cases had high viral load and identical subgenotype, indicating that the viral molecular characteristics play a minimal role in the infection outcome.
Human parvovirus B19 (B19V) causes a broad spectrum of clinical conditions, ranging from mild to life-threatening.1 The acute infection in healthy children leads to erythema infectiosum, which is a mild febrile exanthema, but pediatric patients with hemoglobinopathies often develop transient aplastic crisis (TAC).2 The progression of TAC can lead to severe and even fatal anemia resulting in congestive heart failure, cerebrovascular collapse,3 and acute splenic sequestration.4 Myocarditis, arthritis, nephrotic syndrome5 and fatal bone marrow embolism6 due to B19V have also been reported in patients with sickle cell disease (SCD).
On the contrary, some SCD patients can develop subclinical B19V infection. The reason why they do not represent clinical symptomatology is obscure, but different factors like aplasia duration and severity, fetal hemoglobin concentrations and genetic modifiers have been implicated.7 Moreover, the elevated high substitution rates of B19V have also been regarded as infection modifiers.8
This case report comparison examines the contrasting outcome of B19V infection in two children with SCD during a nosocomial B19V outbreak in the Hemotherapy Center of Ribeirão Preto, Brazil. Despite the identical B19V subgenotypes and high viral load, the patients presented different clinical and laboratory pictures. B19V molecular and genotypic features during acute infection were examined.
Case report 1A four-year-old Afro-Brazilian girl with SCD type SC was subjected to routine blood screening during her visit as an outpatient at the transfusion unit of the Hemotherapy Center of Ribeirão Preto, Brazil (September 3rd, 2010). The patient was asymptomatic and her mother reported no complaints during the past week. The physical examination did not reveal signs of acute infection, such as fever, adenopathy or spleen enlargement.
From the laboratory results (Table 1) three findings were observed: the baseline hemoglobin had stable values (10.5g/dL, baseline 11.0g/dL) on the background of a profound reticulocytopenia (0.3% reticulocytes, range 0.66–2.19%) and thrombocytopenia (122×103μL, baseline 201×103μL). B19V was suspected as a cause for the reticulocytopenia, although the patient was asymptomatic. Therefore, the sample was also quantified for B19V by in-house developed TaqMan PCR (see Methods). The result was positive with 1.2×10/10 viral copies/mL, and although hospitalized for two days the patient remained asymptomatic without development of TAC. The patient was anti-B19V IgG negative (OD=0.115).
Changes of the hematological parameters of the examined children during the acute parvovirus B19 infection.
| Case report number | Blood parameters | Baseline values | Sample no. 1 | Sample no. 2 |
|---|---|---|---|---|
| Case report no. 1 | Hemoglobin | 11.0g/dL | 10.5g/dL | 10.6g/dL |
| Hematocrit | 35% | 33% | 33% | |
| Leukocytes | 12,300 | 6700 | 6500 | |
| Neutrophils | 5700 | 2400 | 2000 | |
| Lymphocytes | 4500 | 2500 | 2600 | |
| Platelets | 201,000 | 122,000 | 201,000 | |
| Reticulocytes | 1% | 0.3% | 1% | |
| Case report no. 2 | Hemoglobin | 7.8g/dL | 4.4g/dL | 8.1g/dL |
| Hematocrit | 26% | 14% | 28% | |
| Leukocytes | 11,900 | 18,200 | 9300 | |
| Neutrophils | 6900 | 2900 | 4000 | |
| Lymphocytes | 3900 | 14,400 | 4500 | |
| Platelets | 232,000 | 398,000 | 434,000 | |
| Reticulocytes | 11.27% | 0.65% | 4.5% | |
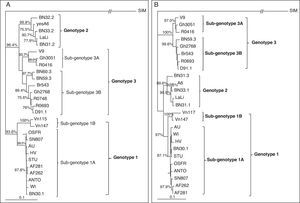
One month later (November 10th, 2010), the patient returned to the Hemotherapy center for a medical evaluation. Her mother did not report complaints or indisposition and the physical examination remained unchanged. Nevertheless, a second blood sample was tested for B19V. The result was negative (rapid viral clearance) and the baseline hemoglobin remained unchanged (10.6g/mL). The platelets and the reticulocytes showed stabilization (Table 1). The phylogenetic analysis of two partial viral genes (VP1 and NS1) revealed that the detected viral isolate belongs to subgenotype 1A of the main genotype 1 (97% bootstrap probabilities for VP1 and 93.6% for NS1, **p<0.01) (Fig. 1A and B).
Phylogenetic analysis of the detected human parvovirus B19 (B19V) isolates from acutely infected and asymptomatic children with sickle cell disease. (A) Rooted NJ tree of the reference B19V genotypes and the sequences obtained from the patients with acute (AF282) and asymptomatic infections (AF262) based on a 442bp fragment of the NS1 region. The simian parvovirus (SIM) is used as an outgroup; (B) Rooted NJ tree of the reference B19V genotypes and the sequences obtained from the patients with acute (AF282) and asymptomatic infections (AF262) based on a 699bp fragment of the VP1 region. Simian parvovirus is also used as an outgroup. The posterior probabilities (above 75%, using 1000 bootstrap replicates) are indicated at the clusters. The statistical evaluation of some important branches was also performed by the Maximum Likelihood method (**p<0.01 and *p<0.05).
A 10-year-old boy with Sβ0-SCD suddenly developed serious hypoplastic anemia on November 8th, 2010 and acute B19V infection was suspected. The patient was pale and as the condition deteriorated progressively, he was hospitalized and treated by emergency blood transfusions (10mL/kg of packed red cells). Additional symptoms included five-day history of pain in the arms and the legs, lethargy and malaise. The day before seeking medical care, the patient had episodes of fever and vomiting. The physical examination showed cervical adenopathy, left upper quadrant tenderness and an enlarged spleen. Post-transfusion, the spleen decreased in size and the blood counts improved.
At the time of the acute infection a blood sample was collected for evaluation of the blood parameters, and for B19V detection. The hematological parameters displayed a significant drop of the hemoglobin (4.4g/dL), reticulocytopenia (0.65%, baseline 11.27%), lympho- (14.4×103, baseline 3.9×103) and leukocytosis (18.2×103, baseline 11.9×103) (Table 1). The B19V TaqMan PCR was positive and demonstrated 1.6×107 viral copies/mL. Although, high this viral load was significantly lower than that of case report no. 1. Nevertheless, this patient developed severe TAC, life-threatening anemia and needed emergency transfusions. After blood transfusion, the hemoglobin was stabilized from 4.4g/dL (November 8th, 2010) to 8.1g/dL (November 10th, 2010) but the TAC continued approximately one week. In this comparative case, the acute infection was connected with severe anemia, as well as life-threatening drop of the hemoglobin and hematocrit on the background of lymphocytosis, leukocytosis and reticulocytopenia. The serologic detection of anti-B19V IgG at the time of the collection of the molecular diagnosis demonstrated negative result (OD=0,201U/mL).
The phylogenetic analysis of partial VP1 and NS1 gene sequences demonstrated that the isolate belongs to subgenotype 1A of genotype 1 (97% bootstrap probabilities by the VP1 and 93.6% by the NS1 gene). This sequence formed one cluster with the B19V isolate from the asymptomatic patient (Fig. 1A and B) with high similarity indicating the identity of the infecting subgenotypes.
The second blood sample obtained at the end of the month (November, 26th) showed a return of the hemoglobin and reticulocyte levels to the patient's baseline. Nevertheless, the sample was positive for B19V with a low viral load (2.6×104copies/mL). The patient did not show indisposition or anemia deterioration.
MethodsSamples collectionSix milliliters of total blood were collected in sterile EDTA tubes (Becton Dickinson, USA) by brachial vein puncture. Plasma was separated by low-speed centrifugation (1426×g/10min). During plasma separation all precautions were taken to avoid inter-sample contamination prior to viral detection. The family relatives of both children gave their informed consent prior to B19V testing.
Serological confirmation of B19V infectionAnti-B19V IgG was detected semi-quantitatively by the Ridascreen® Parvovirus IgG kit (R-Biopharm AG, Germany), following manufacturer's instructions. The antibody titer was expressed in U/mL (negative<0.3U/mL, equivocal 0.3–0.5U/mL and positive>0.5U/mL).
Quantification of B19V loadB19V DNA was extracted by the help of the QIAamp® Viral RNA Mini Kit (QIAGEN, Germany) following manufacturer's instructions. B19V infection was diagnosed by the use of in-house optimized TaqMan real-time PCR as already described by Slavov SN and cols.9 To ensure the analytical verification of the reaction, all appropriate controls were applied, including 99/800 WHO B19 NAT standard (5×105IU/mL, NIBSC, UK). In order to reduce the risk of B19V DNA contamination because of the high B19V viral load, all the diagnostic procedures (DNA extraction, sample application, master mix preparation and PCR) were performed in different laboratory rooms certified for molecular viral detection.
DNA sequencing and phylogenetic analysisThe sequencing was performed in ABI 3130XL Genetic Analyzer (Life Technologies, USA). The phylogenetic analysis was carried out on two partial genes: 442bp fragment of the non-structural region (NS1) and 699bp fragment of the VP1 region, amplified by established primer pairs.10 Multiple alignment was performed by ClustalW (BioEdit, Carlsbad, CA). Neighbor-Joining and Maximum Parsimony trees were reconstructed by the program Phylip v 3.69 (Felsentstain J, University of Washington, USA). Divergences between the clades were estimated by the Kimura-2-parameter using 1000 bootstrap replicates. Only values above 75% were considered as significant and the statistical evaluation of some important branch lengths was also performed by the Maximum Likelihood method (**p<0.01 and *p<0.05).
DiscussionAlthough, B19V is a common pathogen in the general population, its clinical manifestations among children with SCD are poorly defined. SCD is a chronic disease, characterized by repeated vaso-occlusive and aplastic crises frequently triggered by a bacterial or viral infection.11 Despite the fact that most of the patients develop humoral anti-B19V response after the acute infection, it is not uncommon for patients with SCD to establish persistent viral carriage with unknown squeal.9 Although, B19V can persist for prolonged periods of time,12 the detection of high viral loads in plasma is indicative of acute infection, which was observed in both diagnosed children, despite the difference in the clinical picture.
In case report no. 1 (SCD type SC), the acute B19V infection was diagnosed occasionally without clinical symptomatology. The only observation was the profound reticulocytopenia observed during routine blood testing. Nevertheless, due to the high viral load, the lack of symptoms in this patient is unfamiliar. In case report no. 2, a child suffering from SCD type Sβ0 developed acute B19V infection with profound hemoglobin and reticulocyte drop needing emergency transfusions. Additional symptoms like fever, malaise, lethargy and painful crisis were also reported. Why some children with SCD and acute B19V infection do not develop clinical symptoms is unclear. Probably, most important are the variable rates of hemolysis seen in different types of SCD. Some additional factors such as fetal hemoglobin concentration, concurrent α-thalassemia, nutritional status and may be B19V genotype can alleviate the virally induced hematologic effects.7 The presence of asymptomatic B19V infection in patients with SCD has other important aspect. Due to the high viral load, these patients can be responsible for nosocomial B19V outbreaks, which is probably our case as the asymptomatic infection was detected initially, and the severe several days after. Such outbreaks are connected with significant morbidity among individuals with hemoglobinopathies in transfusion centers. Therefore, the identification, characterization and isolation of the asymptomatic cases are essential for prevention of such outbreaks. The acute symptoms in case report no. 2 can be a result from the excessive cytopathic B19V effect on bone marrow progenitors documented in patients with SCD type Sβ0, and their higher hemolysis rates. The examined patient did not exhibit rash and is believed that this could be a result from the antigen excess during acute B19V infection.13 Nevertheless, the observed splenomegaly in this patient can additionally contribute to the profound hemoglobin drop.
The single stranded B19V DNA genome is variable14 and currently is controversial how this can influence the outcome of the infection. Some authors15 indicate subgenotype 1A as a frequent cause of hemolytic disorders and related to blood transfusion therapy, which is supported by the observation of patient no. 2, but not that of patient no. 1. The performed phylogenetic analysis demonstrated that the detected isolates belong to subgenotype 1A (genotype 1). Moreover, both sequences formed one cluster, confirmed by the analysis of two different partial genes (VP1 and NS1) (Fig. 1A and B), demonstrating that the genotypic characteristics probably have limited importance for the outcome of B19V infection.
ConclusionIn summary, the comparison of asymptomatic and symptomatic acute B19V cases in pediatric patients with SCD is of significant importance. On one hand, the current existence of advanced sequence and diagnostic procedures will provide a better understanding of the genetic variability of this common viral pathogen and may modify our understanding of the natural history of B19V infection in children with SCD. On the other, regarding the asymptomatic infections, we may need to change our approach to issues like blood transfusion safety, personal risk of one particular patient with SCD to B19V and finally the education of the patients regarding TAC as a serious and potentially life-threatening complication.
Conflict of interestThe authors have no conflict of interest to declare.
We are grateful to FAPESP, CNPq and INCTC for the financial support. We are also grateful to Virginia Wagatsuma for the technical assistance.