Intravenous therapy is a complex procedure usually requiring the preparation of the medication in the clinical area before administration to the patient. Breaches in aseptic technique may result in microbial contaminations of vials which is a potential cause of different avoidable infections. We aimed to investigate the prevalence and pattern of microbial contamination of single- and multiple-dose vials in the largest pulmonary teaching hospital in Iran.
MethodsIn a period of 2 months, opened single- and multiple-dose vials from different wards were sampled by a pharmacist. The name of the medication, ward, labeling of the vials, the date of opening, and storing temperature were recorded for each vial. Remained contents of each vial were cultured using appropriate bacterial and fungal growth media.
ResultsMicrobial contamination was identified in 11 of 205 (5.36%) of vials. The highest contamination rate was 14.28% for vials used in interventional bronchoscopy unit. The most frequent contaminated medication was insulin. Gram-positive bacteria (81.82%) were more significantly involved than gram-negative ones (9.09%) and fungi (9.09%), with the highest frequency for Staphylococcus epidermidis.
ConclusionsOur data demonstrate that repeated use of vials especially if basic sterility measures are disobeyed can cause microbial contamination of administered products to the patients. Infection preventionists are responsible to train health care workers regarding aseptic techniques and apply guidelines for aseptic handling of intravenous solutions.
Thirty to fifty percent of hospitalize patients receive intravenous therapy which requires the preparation and handling of the medicine before administration to the patient.1 Parenteral medications are usually given out in single- and multiple-dose vials (SDVs and MDVs). A SDV is a vial of liquid medication intended for parenteral administration (injection or infusion) that is meant for use in a single patient for a single case/procedure/injection.2 A MDV is a vial of liquid medication intended for parenteral administration (injection or infusion) that contains more than one dose of medication. MDVs should be dedicated to a single patient whenever possible.3
The revised version of United States Pharmacopeia (USP) Chapter 797 is a comprehensive document that describes standards and procedures to minimize the risk of contamination of compounded parenteral products. The chapter includes evidence-based instructions for pharmacy design, washing, garbing, cleaning, quality assurance, and personnel training and evaluation designed to improve compounding practices in all pharmacies that compound parenteral products.4 However, sterile compounding procedures vary widely across the countries. In our country the majority of the reconstitution of injectable drugs is carried out right before the administration to the patient by the nursing staff. Prevalence of bacterial contamination of SDVs which were used more than once has been reported 5.6% in one of Iranian hospitals.5 Fungal contamination of SDVs and microbial contamination of vials containing preservative were not examined in this report. The information regarding extrinsic microbial contamination of injectable drugs and potentially serious adverse events is slight in our country and still no measure has been taken to improve standards for intravenous therapy. We designed this study to understand the prevalence of contaminations of intravenous medications in our hospital and to design future intervention which could be made by infection control staff to prevent the contaminations of the injectable drugs.
Material and methodsSample takingInjectable drugs were prepared in a room with no special air-conditioning on the ward or unit in which everybody even the patient accompanied person and cleaning staff could enter in and out liberally. Using sterile technique, 3mL of the medication was withdrawn from opened SDVs and MDVs daily by a pharmacist without prior warning. Before sampling, the vials were shaken briskly, and the rubber was swabbed with 70% ethanol. Name, potency and total volume of the vial, clinical ward, labeling or nonlabeling of the vials, the date and time of opening, storage condition, expiration date, and manufacturer name were then recorded.
Laboratory diagnosticEach sample was tested using three methods. (1) 1mL was put into a tube containing 15mL thioglycolate broth and incubated at 37°C for 10 days. The broth was visually examined every day and subcultured onto blood, chocolate and sabouraud dextrose agar plates every other day within 10 days or any time that the appearance seemed turbid.5 (2) 1mL was centrifuged (3000rpm, 15min), then the pellete was inoculated into blood, chocolate, MacConkey's and sabouraud dextrose agar. Two first media were evaluated after 48h and the sabouraud dextrose agar was evaluated after 18 days. (3) 1mL was filtered using 0.45μm filters and the filters were placed onto blood agar plates. Plates were incubated for 48h at 37°C and evaluated for bacterial growth. They were stored for fungal growth for 18 days. The bacterial isolates were identified using Gram's staining and standard biochemical methods.
Statistical analysisDescriptive statistics were used to detail the distribution of contaminated vials and the contaminating microorganism. Due to the non-normal distribution of values, Mann–Whitney test was used to determine the relation of vial contamination and the date of opening of vial. The relation of type of vial and contamination rate was evaluated using Fisher's exact test. A P value of less than 0.05 was considered as statistically significant. Statistical analysis was performed using SPSS version 16.0.
ResultsA total of 205 vials (165 SDVs and 40 MDVs) were tested from 18 wards and units, with 29 medication types. Table 1 shows sampled medications from different wards/units. All vials were being used within their expiration period, and no vial had expired. No statistical difference was observed between contamination rate and the number of days that the vials were opened. A total of 115 (56.10%) vials were kept at room temperature, the rest at 4°C.
Medication, name of ward/unit, and number of sampled vials.
| Medication | Ward/unit | Respective number of sampled vials |
|---|---|---|
| Acetylcysteine 200mg/10mL | Pediatric | 1 |
| Aminophyline 250mg | Emergency, tuberculosis ICU | 1, 1 |
| Atracurium 50mg/5mL | Interventional bronchoscopy | 1 |
| Atropin 0.5mg/mL | Interventional bronchoscopy | 1 |
| Blood cardioplegia | Operation room and anesthesiology | 1 |
| Bupivacaine 100mg/20mL | Operation room and anesthesiology | 3 |
| Ceftazidim 2g | Emergency | 1 |
| Dextrose 5% | Internal (3), internal (9), emergency, surgical ICU | 4, 3, 1, 1 |
| Dextrose 3.33%+sodium chloride 0.3% | Internal (9), pediatric, emergency | 1, 5, 2 |
| Ganciclovir 500mg | Transplant | 2 |
| Heparin 5000U/mL | Internal (3), internal (4), internal (9), tuberculosis (5), pediatric, oncology, emergency, surgical ICU, sleep, surgery | 1, 1, 1, 1, 1, 1, 1, 1, 1, 2 |
| Hydrocortisone 100mg | Tuberculosis ICU | 1 |
| Insulin NPH 100U/mL | Internal (3), internal (4), internal (9), tuberculosis (5), tuberculosis (6), oncology, CCU, emergency, surgical ICU, post CCU, surgery, transplant | 1, 1, 2, 2, 1, 1, 1, 2, 1, 1, 2, 1 |
| Insulin regular 100U/mL | Internal (3), internal (4), internal (9), tuberculosis (5), tuberculosis (6), oncology, CCU, emergency, surgical ICU, tuberculosis ICU, medical ICU, operation room and anesthesiology, surgery, transplant | 4, 1, 2, 2, 2, 1, 1, 3, 1, 1, 1, 1, 3, 1 |
| Ketamine hydrochloride 50mg/mL | Interventional bronchoscopy, operation room and anesthesiology | 2, 1 |
| Lidocaine 2% | Interventional bronchoscopy | 1 |
| Magnesium sulfate 20% | Internal (3), internal (4), internal (9), tuberculosis (6), emergency, surgical ICU, tuberculosis ICU, medical ICU, operation room and anesthesiology | 1, 1, 1, 1, 2, 7, 1, 1, 1 |
| Meropenem 1g | Surgical ICU | 2 |
| Methylprednisolone 500mg | Internal (9), pediatric, emergency | 1, 2, 3 |
| Morphine | Internal (3) | 1 |
| Omnipaque 240mg/mL | Radiology | 1 |
| Potassium chloride 15% | Internal (4), internal (9), tuberculosis (5), tuberculosis (6), emergency, surgical ICU, tuberculosis ICU, medical ICU, Operation room and anesthesiology, transplant | 1, 3, 2, 1, 1, 9, 2, 6, 1, 3 |
| Propofol 1% (w/v) | Interventional bronchoscopy | 1 |
| Sodium chloride 0.45% | Emergency | 1 |
| Sodium chloride 5% | Internal (3), emergency, tuberculosis ICU, medical ICU | 2, 1, 1, 1 |
| Sodium chloride 0.9% | ||
| Internal (3), internal (4), internal (9), tuberculosis (5), tuberculosis (6), pediatric, oncology, CCU, emergency, heart clinic, surgical ICU, surgery, transplant | 8, 6, 5, 1, 3, 5, 2, 3, 2, 1, 2, 10, 1 | |
| Succinylcholine 500mg | Interventional bronchoscopy | 5 |
| Thiopental sodium 1g | Interventional bronchoscopy, Operation room and anesthesiology | 2, 1 |
| TNG 100mg/mL | Interventional bronchoscopy | 1 |
Bacterial contamination was identified in 11 of 205 (5.36%) of vials. Contamination rate for SDVs and MDVs were 4.85% and 7.50% respectively. There was no significant difference in the frequency of contamination of different type of vials. Contaminations were found in three internal wards, emergency ward, Intensive Care Unit (ICU), transplant unit, and interventional bronchoscopy unit. The highest contamination rate was 14.28% (2/14) for vials used for interventional bronchoscopy unit and the lowest was 4.54% (1/22) for vials used for one of the internal wards which named as internal ward 3.
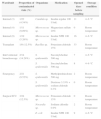
The most frequent contaminated solution was insulin NPH 100U/mL. No mixed contamination was detected in any of vials. Gram-positive and gram-negative bacteria were involved in 9 (81.82%) and 1 (9.09%) of contaminations, respectively. Fungal contamination was detected in one sample (9.09%). Therefore, gram-positive bacteria were more significantly involved (p<0.05) in vial contaminations. Most commonly identified microorganisms were part of the normal commensally flora with the highest frequency for Staphylococcus epidermidis (4/11 or 36.36%). Table 2 shows the distribution of contaminated vials in different wards/units of the hospital along with the contaminating microorganism and the characteristics of the vials.
Distribution and frequency of contaminated vials in different wards/units, isolated bacteria, and the characteristic of contaminated vials.
| Ward/unit | Proportion of contaminated vials (%) | Organisms | Medication | Opened days before sampling | Storage condition |
|---|---|---|---|---|---|
| Internal (3) | 1/22 (4.54%) | Candida sp. | Insulin regular 100U/mL | 11 | 4–6°C |
| Internal (4) | 1/11 (9,09%) | Micrococcus sp. | Magnesium sulfate 20% | 0 | Room temperature |
| Internal (9) | 1/19 (5.26%) | Micrococcus sp. | Insulin NPH 100U/mL | 16 | 4–6°C |
| Transplant | 1/8 (12.5%) | Bacillus sp. | Potassium chloride 15% | 33 | Room temperature |
| Interventional bronchoscopy | 2/14 (14.28%) | S. epidermidis | Succinylcholine 500mg | 7 | 4–6°C |
| S. epidermidis | Succinylcholine 500mg | 8 | 4–6°C | ||
| Emergency | 2/21 (9.52%) | S. epidermidis | Methylprednisolone 500mg | 2 | Room temperature |
| S. epidermidis | Dextrose 3.33%+sodium chloride 0.3% | 0 | Room temperature | ||
| Surgical ICU | 3/24 (12.5%) | Micrococcus sp. | Potassium chloride 15% | 0 | Room temperature |
| Nocardia sp. | Sodium chloride 0.9% | 2 | Room temperature | ||
| E. coli | Insulin NPH 100U/mL | 9 | 4–6°C | ||
Six (54.54%) of contaminated vials were not marked with patient's name, which indicated that they were probably used for more than one patient.
DiscussionOur data show a contamination rate of 5.36% with bacteria and fungi, in the content of the SDVs and MDVs used in different wards/units of a pulmonary teaching hospital in Iran. In principle, preparation, storage and transportation of Compounded Sterile Preparations (CSPs) require aseptic conditions and trained personnel. USP Chapter 797 is a comprehensive document that describes standards and procedures to minimize the risk contamination of CSPs.6 But standards for CSPs in developing countries may be limited by lack of resources (trained personnel and facilities). Non-standard preparation and handling of vials (which are assumed to be sterile) result in contamination rates, ranging from 0% to 27%.7
SDVs are preservative free vials which are intended to be used only once. Puncturing SDVs multiple times and pooling preservative free solutions may cause the potential contamination risk, possibly leading to severe infections in patients.8,9 In the current study 165 of 205 vials (80.49%) were SDVs that were used as multiple-dose vials. This finding is more than reported percentage of 50% in the literature.10,11
On the other hand MDVs contain antibacterial preservatives and may be used more than once when preparation and storage is according to the manufacturer's recommendations. (e.g., insulin, some heparin, lignocaine and octeotride products).8 If MDVs must be used for more than one patient, they should not be kept or accessed in the immediate patient treatment area. This is to prevent inadvertent contamination of the vial through direct or indirect contact with potentially contaminated surfaces or equipment that could then lead to infections in next patients. If a MDVs enters the immediate patient treatment area, it should be dedicated to that patient only and discarded after use.3
It must be noticed that a preservative does not prevent non-bacterial and non-fungal contaminations (e.g., viral, protozoa, and prion pathogens) and does not prevent growth of microorganisms in low temperature.7 MDVs remain prone to bacterial contamination and the use of them has been reported to be a potential source of infections in different studies.12–17 Our study also shows that microorganisms can survive in the presence of a preservative as 3 of the contaminated vials were insulin. The sterility of multidose insulin vials was determined up to 50 days by Rathod et al. They showed bacterial contaminations in 8 of 69 insulin vials and concluded that antibacterial preservatives were more effective at room temperature than at refrigerator temperature.18 Other study by Jackson et al. verified that prefilled insulin syringes remained sterile for up to one month after preparation when they were prepared using good aseptic technique and stored in the patient's refrigerator.19
The sterility of a CSP is directly related to employment of the best practice and quality standards. Safe preparation and handling of CPSs within a properly operating unidirectional airflow in an ISO class 5 clean room in accordance with USP chapter 797 requirements is the best way to avoid bacterial or fungal contamination.20,21 The standardization then the centralization of the preparations and reconstitution of CPSs by infection prevention and control experts makes it possible to reduce contamination risk related to injectable drugs.22 Although the requirements of USP chapter 797 may appear complicated, expensive, and even unattainable in developing countries, the first step to establish quality standards could be made through training of health care workers according to recommendations from CDC and WHO.23,24 The most applied recommendations include dating MDVs after opening and discarding them on the manufacture's dates, discarding SDVs after opening, and emphasizing the need for proper aseptic technique. Simple aseptic techniques that could be implemented in each hospital setting may be summarizes as: performing hand hygiene before preparing medications for administration; using gloves, face mask, and avoidance of talking during an injection;25 wiping the outsides of vials with 70% isopropyl alcohol swabs before opening and aspirating the contents of vials using a 5p.m. filter straw26; and considering practical guidelines for lipid based emulsions that supports bacterial growth such as propofol.27 Moreover reconstitution of high risk level intravenous treatments by a centralized hospital pharmacy service under sterile conditions is the next measure to reduce both infection risk and cost due to discarding expensive vials.
In conclusion infection preventionists in developing countries should improve sterile compounding of injectable products in a hospital. Although implementation of the guidelines from professional pharmacy organizations, such as the American Society of Health-System Pharmacists (ASHP) and the National Association of Boards of Pharmacy (NABP) guarantees patient safety, understanding the problems and limitations in a hospital is essential to develop a regional standard procedure.
Conflict of interestThere is no conflict of interest that should be disclosed by the authors.