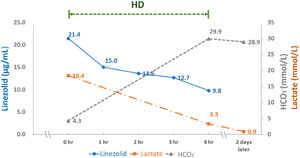
Severe lactic acidosis, a mitochondrial toxicity caused by the recommended standard dosage of linezolid (LZD), may occur in patients with impaired renal function. We describe an adult male who underwent kidney transplantation with stably impaired renal function, severe dyspnea, and abdominal discomfort. He received a standard oral dose of LZD (600 mg twice daily) and azithromycin for three weeks with a reduced immunosuppressant dose due to pulmonary non-tuberculosis mycobacterial infection. He was alert and afebrile, with a blood pressure of 140/60 mmHg. Pertinent laboratory data showed: pH 7.12, PaCO2 13.6 mmHg; HCO3- 4.3 mmol/L and serum lactate 18.4 mmol/L. His trough serum LZD concentration reached toxic levels (21.4 μg/mL). With hemodialysis, his clinical symptoms improved, with a decline in serum LZD (9.8μg/mL) and lactate (3.2 mmol/L). Chronic standard dose LZD in patients with impaired renal function can lead to life-threatening lactic acidosis, especially in coexisting conditions that reduce LZD metabolism.
Linezolid (LZD), which inhibits bacterial protein synthesis by blocking the fusion of ribosomal subunits, has been increasingly used for multidrug-resistant Gram-positive microorganisms and as an alternative treatment for mycobacterial infection.1 In pharmacokinetic studies with healthy adults, the time to reach maximum concentration (Tmax) after oral administration of LZD was approximately 1 to 2 hours.2,3 Seventy percent of LZD is converted to inactive compounds in the liver, and 30% is eliminated via the renal route, with an elimination half-life of 5 to 7 hours with the therapeutic trough concentration being 2–8 μg/ml.2 In patients with impaired renal function, its elimination half-life increased from 6.1 to 8.4 hours.4 Chronic use of LZD may cause adverse side-effects such as hepatic toxicity, peripheral neuropathy, and myelosuppression.5 Mitochondrial toxicity of LZD causing severe lactic acidosis is potentially fatal, usually related to its higher or toxic trough serum concentration.6,7
LZD is currently recommended as a fixed dose of 600 mg twice daily, irrespective of renal function.8 However, impaired renal function is a well-known and important factor in patients with LZD-induced lactic acidosis.9 Nevertheless, standard dose LZD-induced lactic acidosis in patients with renal impairment may still be less well known. Herein, we describe a kidney transplant recipient with nephrotic syndrome and impaired renal function (chronic kidney disease (CKD) stage IV) on a minimal dose of immunosuppressant who developed life-threatening lactic acidosis associated with a toxic trough serum LZD level three weeks after repeated administration of oral standard doses of LZD and azithromycin.
Case reportsA 54-year-old man with lupus nephritis in a uremic state had received cadaveric kidney transplantation and had been on immunosuppressants for 12 years with CKD stage IV and nephrotic syndrome presented to the emergency department with worsening dyspnea and abdominal discomfort. Three weeks earlier, he developed a newly diagnosed pulmonary non-tuberculosis mycobacterial (NTM) infection, treated with 600 mg LZD twice a day and 250 mg azithromycin daily coupled with a reduced dose of immunosuppressants including prednisolone (5 mg daily), mycophenolic acid (180 mg daily), and sirolimus (0.25 mg daily). His serum creatinine was around 3.2 mg/dl, with an estimated glomerular filtration rate (eGFR) of 18 ml/min.
On physical examination, he was alert and oriented. His blood pressure was 140/60 mmHg, heart rate was 122 beats/min, and body temperature was 35.8°C. His blood oxygen saturation on pulse oximetry was 100% with 40% oxygen support. Pale conjunctiva and tachypnea (28-32 breath rate/min) with rales and bilateral grade II pitting edema were noted. Blood laboratory data showed: hemoglobin 6.5 g/dL, serum sodium 135 mmol/L, potassium 5.8 mmol/L, chloride 99 mmol/L, lactate 18.4 mmol/L, anion gap (AG) 31.7 mmol/L, SCr 3.5 mg/dL, blood urea nitrogen 60 mg/dL, albumin 3.1 g/dL, c-reactive protein 1.5 mg/dL, and normal liver profile.
Atrial blood gas revealed pH 7.122, PaCO2 13.6 mmHg, PaO2 87.8 mmHg, HCO3- 4.3 mmol/L. Pulmonary radiography showed increased infiltration in both lung fields. Echocardiography revealed normal ventricular function. His serum trough LZD level was 21.4μg/mL, much higher than the therapeutic range (2- 8μg/mL). With emergent hemodialysis (HD) for four hours and withdrawal of LZD, his clinical symptoms rapidly resolved along with a decline in serum LZD (9.8 μg/mL) and lactate (3.3 mmol/L) (Fig. 1). The patient's hospital course was uneventful. LZD and azithromycin were then reduced to 600 mg and 250 mg daily, respectively.
Successful remission of pulmonary NTM without recurrence of lactic acidosis was noted six months later. His serum creatinine was 3.8 mg/dl yet dialysis.
DiscussionThis kidney recipient with CKD stage 4 and nephrotic syndrome manifested with progressive dyspnea and abdominal discomfort associated with severe lactic acidosis. With stable hemodynamics, normal cardiac and liver function, and the absence of sepsis, type B lactic acidosis caused by mitochondrial dysfunction was more favorable than type A lactic acidosis due to poor tissue perfusion and oxygenation. There was no evidence of malignancy, making drug-induced lactic acidosis more likely. Upon reviewing his medication history, LZD-induced lactic acidosis was highly suspected. A toxic trough serum level of LZD and elimination of LZD by hemodialysis (HD) correcting lactic acidosis supported the diagnosis.
LZD-induced lactic acidosis is alarming because of its high mortality rate, ranging from 15% to 43%. Its incidence rate varies from 2% to 33%.10 The clinical manifestations of LZD-induced lactic acidosis are non-specific, including gastrointestinal discomfort (33%), change in mental status (27%), and dyspnea (19%), which make early detection challenging.10 Without therapeutic drug monitoring (TDM), it would be difficult to clarify the causality between LZD and lactic acidosis, as other risk factors of lactic acidosis often coexist.
As a rule, lower dose adjustment in renal impairment is usually only required if the kidney accounts for less than 30% of drug elimination, such as LZD.2,11 In uninfected patients across the whole spectrum of renal dysfunction, pharmacokinetic analysis of oral single-dose LZD (600 mg) demonstrated that total LZD clearance did not decrease as renal function declined.4 In infected patients with chronic LZD use, renal function impairment was independently associated with elevated LZD trough concentration and increased toxicity.12,13,14 This suggests that chronic standard LDZ use in reduced renal clearance may cause progressive LZD accumulation. The established risk factors for LZD-induced lactic acidosis, such as advanced age, prolonged treatment duration over two weeks, and genetic susceptibility may be present in patients with impaired renal function.15 In addition, comorbid conditions, such as hypoalbuminemia and decreased lean body mass, reduced LZD volume distribution, and p-glycoprotein-mediated drug-drug interaction with macrolides (azithromycin in this case), which may reduce LZD metabolism, could also worsen LZD toxicity, as shown in our patient.16-18 Apart from adult patients, the onset of LZD-induced lactic acidosis appears much earlier in pediatric cases, which makes detection more urgent.19 Accordingly, LZD should be optimized with TDM-guided dose adjustment in patients with renal dysfunction and coexisting comorbid conditions.20
The key treatment for LZD-induced lactic acidosis is to rapidly eliminate the accumulated drug in addition to acidosis correction. Given LZD's low molecular weight 337.35 Da, 0.5–0.8 L/kg volume of distribution, and 31% protein bound fraction, it is considerably dialyzable. With HD for four hours, a rapid decline in serum LZD from 21.4 to 9.8 μg/mL was noted in this patient, indicating that 54% of LZD was eliminated, and its half-life was reduced to less than 4 hours. Accordingly, HD shortened the recovery time of LZD intoxication, similar to previous reports.21
In summary, our case highlights that chronic standard-dose LZD-induced severe lactic acidosis may occur in patients with impaired renal function because of the coexistence of comorbidities that reduce LZD metabolism. Individualized dose adjustment and careful TDM of LZD are necessary in high-risk patients.
FundingThis research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Ethics approval and consent to participateNot applicable.
Consent for publicationWritten informed consent was obtained from the patient.
We thank the patient for kindly consenting to the publication of this case.