JC virus (JCV) is a member of the Polyomaviridae family and is associated to a severe disease known as progressive multifocal leukoencephalopathy, PML, which is gradually increasing in incidence as an opportunistic infection among AIDS patients. The present study aimed to investigate the occurrence of JCV among HIV-1 carriers including their types and molecular subtypes and the possible association with disease. Urine samples from 66 HIV-1 infected subjects were investigated for the presence of the virus by amplifying VP1 (215bp) and IG (610bp) regions using the polymerase chain reaction. JCV was detected in 32% of the samples. The results confirmed the occurrence of type B (subtype Af2); in addition, another polyomavirus, BKV, was also detected in 1.5% of samples of the HIV-1 infected subjects. Apparently, there was no significant difference between mono- (HIV-1 only) and co-infected (HIV-1/JCV) subjects regarding their TCD4+/TCD8+ lymphocyte counts or HIV-1 plasma viral load. Self admitted seizures, hearing and visual loses were not significantly different between the two groups.
Polyomaviruses are small agents which infect a variety of species including man, non-human primates, cattle, rodents, rabbits, and birds. Two human polyomaviruses are important pathogens, JC virus (JCV) and BK virus (BKV), as they are able to cause a severe, usually fatal, neurological disease (progressive multifocal leukoencephalopathy, PML) and renal infection in immunosupressed patients, respectively.1–3 The viruses share approximately 72% of nucleotide homology and a high degree of identity (80%) of amino acid sequence of a capsid protein, VP1.4,5 JCV infects the human host and establishes a persistent replicative cycle (including latency) following the acute infection, which favors its dissemination within human populations and its carriage through generations.
Although JCV is found to infect more than 70% of the adult human population as a productively persistent infection, PML is a rare disease.5–9 It is a manifestation that follows malignant conditions, as well as a consequence of primary or secondary immunodeficiencies, particularly in acquired immunodeficiency syndrome (AIDS). The emergence of human immunodeficiency virus 1 (HIV-1) infection elicited an additional interest in JCV with the frequent finding of HIV-1 carriers co-infected with JCV.10 The incidence of PML is gradually increasing among AIDS patients, initially as a consequence of the increase of HIV-1 infections, and secondly, as an increase in life expectancy as a result of better antiretroviral drugs.10 JCV infects HIV-1 carriers and is one of the major opportunistic infections of the central nervous system.11–15 In our previous study we described JCV and BKV infections among Amerindian tribes, Afro-descendant communities, as well as in the urban area of Belém, state of Para, north of Brazil.16 The results showed a large genetic variability of strains circulating in the region, infecting a large group of individuals. The presence of European, Asiatic, and African subtypes associated to the ethnic origin of the population samples investigated highlighted the idea that JCV is a good marker for studying the early migration of human populations, reflecting their early and late history.
The present study aimed to describe the occurrence of JCV, including their types and molecular subtypes among HIV-1 carriers and the possible association with neurological disease.
Materials and methodsPopulation examinedA cross-sectional study was performed, using a convenience sample which included 66 HIV-1 infected persons. A single urine sample was obtained from HIV-1 infected persons residing in the city of Belém, attending the University Hospital João de Barros Barreto (HUJBB), for their routine clinical care, and laboratory follow-up determinations of TCD4+/TCD8+ lymphocyte counts and HIV-1 plasma viral load (HPVL). All patients were receiving antiretroviral therapy (ART) at the time of sample collection. The duration of ART ranged from one to seven years. Half of them were on ART for no more than five years, with mean and median values of four years. The project was approved by the Ethics Committee of the University Hospital Joao de Barros Barreto (Protocol Number 2092/05). The participants were briefed about the project and those who accepted to take part signed an informed consent. All patients were adults (18 years or older) and were capable to provide answers to a questionnaire which included questions regarding demographic, social and cultural aspects of their life style. The patients were also asked to inform, over common neurological events which are usually considered as baseline symptoms associated to JCV infection, at least once, since their initial diagnosis of HIV-1 infection.
Quantification of HPVL and TCD4+/TCD8+ lymphocyte countsHPVL was determined by the bDNA method (VERSANT® HIV-1 RNA 3.0 Assay bDNA “Versant Assay”, Siemens, USA) following the manufacturer's directions. Whole blood samples were processed within four hours of collection for the determination of TCD4+ counts by flow cytometry (FacsCount, Becton & Dickinson, San Jose, CA, USA) using the FacsCount™ Reagents immunomonitoring kit, following the protocol recommended by the manufacturer (Becton Dickinson, San Jose, CA, USA). Both procedures were routinely conducted at the Virology Laboratory, which is one of the National Reference Laboratory for the two determinations.
Urine sample collection and DNA extractionUrine samples (50mL) were collected in specific vial collectors and transported to the Virology Laboratory of the Institute of Biological Sciences of the Universidade Federal do Para (ICB/UFPA) and stored at −20°C until assaying for JCV infection. The urine samples were centrifuged at 5000rpm for 15min, the urinary sediment washed three times with sterile saline solution and the cell pellet used for DNA extraction according to the protocol using the EZ-DNA kit (Gentra Systems, Inc., Minneapolis, MN, USA), as previously described.16
Molecular analysisPolymerase chain reaction – PCR was performed for amplification of the VP1 gene (215bp) and the genomic region IG (610bp) of JCV, using a thermocycler (Mastercycler Personal, Eppendorf, Germany), and the amplified products were subjected to purification for subsequent sequencing analysis of the nucleotide bases, as previously described.16
Sequencing and subtype analysisThe amplified products of the IG region were used for determining genetic relationships among the strains detected and those recorded in Genbank database. The amplified fragments were submitted to a direct sequencing assay (both forward and reverse) according to the protocol of the ABI Prism Dye Terminator Cycle Sequencing Ready Kit (Life Technologies, Foster City, CA, USA) and the products were loaded on the ABI Prism 310 DNA Sequencer (Life Technologies, Foster City, CA, USA). The nucleotide sequences were used together with other JCV sequences available in the Genbank as previously described.16 Nucleotides sequences obtained in the present study are available in the Genbank databases under the accession numbers KR062063, KR062064 and KR062065 and were submitted to Basic Local Alignment Search Tool – Blast (http://blast.ncbi.nlm.nih.gov/Blast.cgi) aiming to identify the virus subtype.
Statistical analysisThe frequency of HIV-1/JCV co-infection was estimated by direct counting. To determine whether the variables TCD4+, TCD8+ and HIV-1 viral load followed a normal distribution, D’Agostino's test was used, and the mean values of the laboratory markers were compared using ANOVA. A p-value <0.05 was considered statistically significant using the BioEstat 5.0 software.17
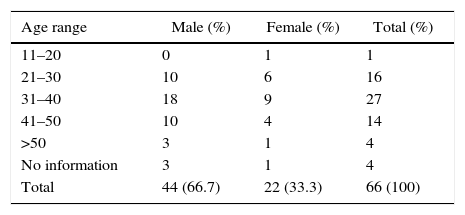
ResultsJCV infection was investigated in 66 urine samples of HIV-1 carriers, outpatients and inpatients, attending the University Hospital. Table 1 shows that most of the patients were male (66.7%), within the age range of 21–50 years old (86.4%).
Amplification of VP1 was obtained in 21 (32%) samples (18 males and 3 females) and 11 of them amplified the two regions, VP1 and IG. Sequencing of nine samples from IG region submitted to Basic Local Alignment Search Tool – Blast (http://blast.ncbi.nlm.nih.gov/Blast.cgi) were identified as belonging to type B, subtype Af2, with 100% identity, confirming the JCV/HIV-1 co-infection. The molecular analysis also showed the amplification of a nucleotide sequence of 541bp, characteristic of BKV polyomavirus, showing 97.4% identity with KOM-3 line and a co-infection BKV/HIV-1 in 1.5%.
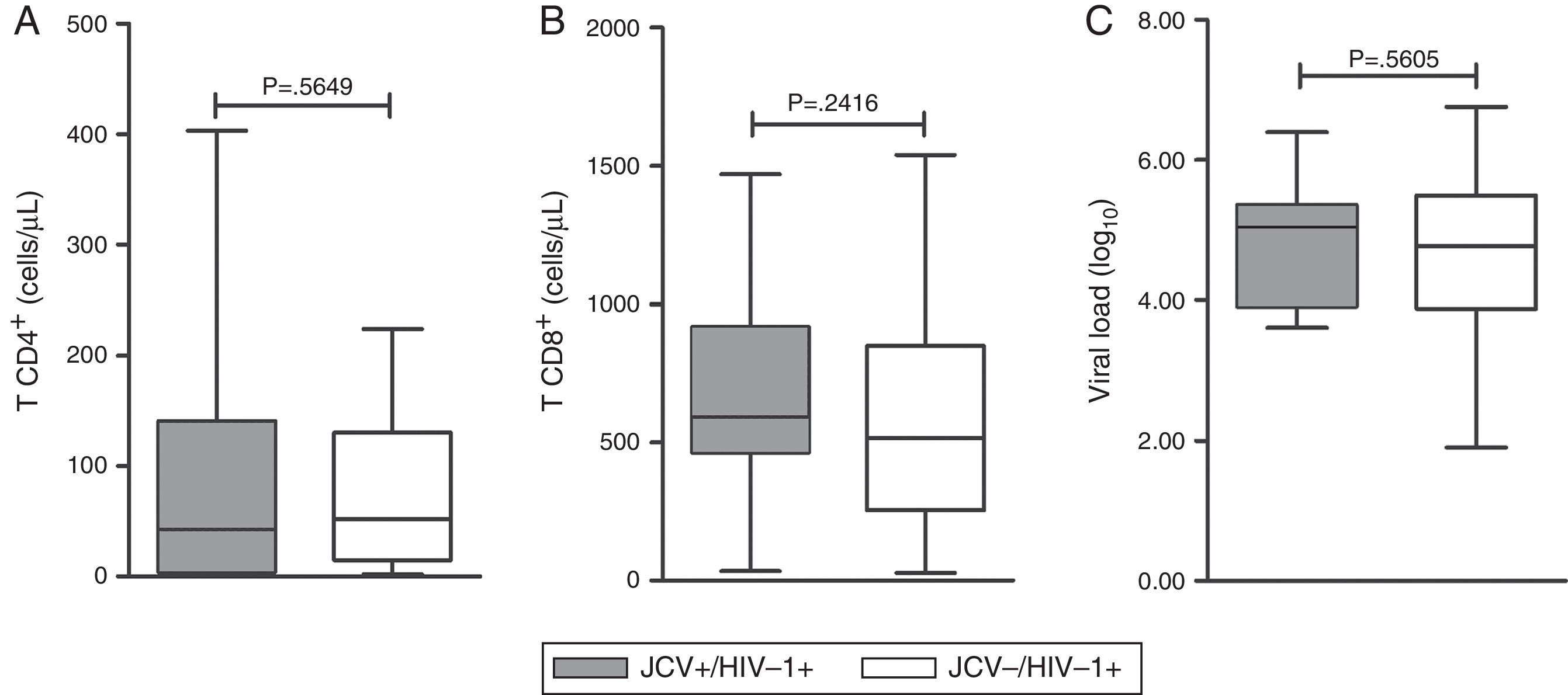
TCD4+ and TCD8+ lymphocyte counts and HPVL (Fig. 1) were compared between the mono-infected (HIV-1 only) and co-infected (JCV/HIV-1) patients. The average counts of TCD4+ were 130.61 and 105.25cells/mL (p=0.5649), and the values for TCD8+ were 589.97 and 679.66cells/mL (p=0.2416), respectively. The mean values for HPVL (log10) were 4.563 and 4.867 (p=0.5605), respectively (Fig. 1).
Neurological events were investigated regarding the presence of seizures, as well as, hearing and vision losses (Table 2). The presence of these baseline symptoms at least once after laboratory diagnosis of HIV-1 infection were admitted by 46.3% and 52.6%, of the mono and double infected individuals, respectively, but the difference between the frequencies was not significant (p=0.860).
Frequency of self-admitted neurological alterations among HIV-1 carriers and co-infected with JCV.
| Clinical symptoms | HIV-1 carriers (%) | JCV/HIV-1 coinfected (%) | Total |
|---|---|---|---|
| With neurological symptoms | 19 (46.3) | 10 (52.6) | 29 (48.3) |
| Without neurological symptoms | 22 (53.7) | 9 (47.4) | 31 (51.7) |
| Total | 41 (100) | 19 (100) | 60 (100) |
χ2=0.031; p=0.860.
The information obtained from HIV-1 carriers were sufficient to establish the socio demographic variables and indicate possible trends of the infection. The age range observed (20–60 years) was in agreement with the epidemic in Brazil.18 Similar results were seen in the USA with patients with non-Hodgkin's lymphoma, HIV-1 negative patients,19 and healthy Koreans.20–23 It is believed that JCV infection occurs early in life and therefore it is necessary to monitor the upsurge of disease among healthy individuals or with associated pathologies within different population groups as the virus prevalence is usually high.5,6,8,9
Infection was defined by the presence of two codifying genomic regions, VP1 and IG, which have been fairly correlated with seroepidemiological information among different populations.23–27 The variation of prevalence rates are also commonly observed among HIV-1 carriers. The present work showed 32% prevalence of co-JCV/HIV-1 infection, which is similar to that reported by others among HIV-1 carriers with or without LPM.7,8,28–32 The search for the virus in other biological fluids including urine, plasma, liquor and cerebral tissue did not increase significantly the prevalence of JCV.30
Higher prevalence rates have been reported in Brazil in the CSF of HIV-1 carriers,33 in Germany, in the blood,34 in France, in brain tissue and blood35,36 and in Italy, where very high and very low rates were described in the CSF of HIV-1 carriers.37,38
Co-infection with BKV is also a commonly reported finding among HIV-1 infected persons.8,39,40 There was only one BKV/HIV-1 co-infected person in the examined group, in contrast to what has been reported by other. The presence of BKV has been previously reported in our geographical area in Afro-descendants living in the region of Rio Trombetas, Para,6 among renal patients and the general population of urban centers.41 The low prevalence of BKV indicates its low levels of dissemination.
HIV-1 infection was more frequent among males, which is consistent with the overall epidemic in Brazil.18 The observation of sex and age distribution of HIV-1 carriers or not, were described by seven laboratories from England and Wales,7 among aborigenes from Central Africa (pigmies and Bantus), in patients from general hospital in Australia, including immunocompromised persons,27 and in Brazil40 but there were no statistically significant differences of prevalence between sexes.
TCD4+ and TCD8+ lymphocyte counts, as well as, HIV-1 viral load were not different between mono- and double-infected subjects, which is a common finding,8,39,40,42 suggesting that the frequency of JCV shedding may not be influenced or altered by the immunodeficiency process, but it remains a matter of controversy yet to be defined.
The investigation of possible disease among the examined group was approached by a self-admitted present or past common symptoms of major perception, including seizures, hearing and vision losses. There was no difference between the frequencies found among the mono- and double-infected groups. Although JCV is a major opportunistic infection of the CNS,11–15,43 PML is not commonly reported in Brazil.40 Either we are not performing the correct diagnosis of minor and major symptoms of PML, or reactivation of the virus, although a common feature, is without clinical consequences in HIV-1 infected persons in the North and Southeast regions of the country.
Until now, there have been few reports of JCV and BKV of either mono-infection or HIV-1 co-infection in Brazil. The results obtained, so far, show a large frequency of excretion of JCV and sometimes of BKV. The clinical consequences of the infections with both viruses should not be ignored and should deserve a deeper look into the actual clinical and epidemiological picture, including the improvement and dissemination of laboratory diagnosis of both infections.
FundingThe present study was fully supported by grants from the STD/AIDS and Hepatitis Department, Ministry of Health/UNESCO, and the Brazilian National Council for Scientific and Technological Development (CNPq).
Conflicts of interestThe authors declare no conflicts of interest.
We thank the subjects and send them our best wishes.