Invasive mold disease is an important complication of patients with hematologic malignancies, and is associated with high mortality. A diagnostic-driven approach has been an alternative to the classical empiric antifungal therapy. In the present study we tested an algorithm that incorporated risk stratification using the D-index, serial serum galactomannan and computed tomographic-scan to guide the decision to start antifungal therapy in neutropenic patients.
Patients and methodsBetween May 2010 and August 2012, patients with acute leukemia in induction remission were prospectively monitored from day 1 of chemotherapy until discharge or death with the D-index and galactomannan. Patients were stratified in low, intermediate and high risk according to the D-index and an extensive workup for invasive mold disease was performed in case of positive galactomannan (≥0.5), persistent fever, or the appearance of clinical manifestations suggestive of invasive mold disease.
ResultsAmong 29 patients, 6 (21%), 11 (38%), and 12 (41%) were classified as high, intermediate, and low risk, respectively. Workup for invasive mold disease was undertaken in 67%, 73% and 58% (p=0.77) of patients in each risk category, respectively, and antifungal therapy was given to 67%, 54.5%, and 17% (p=0.07). Proven or probable invasive mold disease was diagnosed in 67%, 45.5%, and in none (p=0.007) of high, intermediate, and low risk patients, respectively. All patients survived.
ConclusionA risk stratification using D-index was a useful instrument to be incorporated in invasive mold disease diagnostic approach, resulting in a more comprehensive antifungal treatment strategy, and to guide an earlier start of treatment in afebrile patients under very high risk.
Invasive mold disease (IMD) is an important complication of patients with hematologic malignancies, and is associated with high mortality.1,2 Strategies to reduce the incidence and morbidity associated with IMD include the use of antifungal prophylaxis and empiric antifungal therapy.3,4 The latter is considered standard of care in patients with persistent or recurrent fever and neutropenia, but since fever is a non-specific manifestation and may have other etiologies (including uncontrolled occult bacterial infection, viral infection and drug fever),4 a significant number of patients receive antifungal therapy unnecessarily. A diagnostic-driven approach has been studied as an alternative to the empiric therapy. In this strategy, other parameters such as serial serum Aspergillus galactomannan antigen (s-GMI) and computed tomography (CT) of the chest and sinuses are used to trigger the initiation of antifungal therapy.5–7 A major risk factor for IMD in hematologic patients is severe (<100/mm3) neutropenia lasting >10–15 days,8–10 and clinicians rely on a certain duration of neutropenia above which an IMD is suspected. A major limitation of this strategy is the lack of a parameter that measures both the intensity and the duration of neutropenia. We developed an index (D-index) that combines the intensity and duration of neutropenia, calculating the area over the neutrophil curve. The index was tested retrospectively in patients with acute myeloid leukemia (AML), and showed a good discriminatory performance in identifying patients with IMD. A cut-off was derived, and showed good sensitivity, specificity, and a very high negative predictive value (97–99%).11 The high negative predictive value of this index is very similar to that obtained with s-GMI and 1,3-β-d-glucan (BDG) in febrile neutropenic patients.12,13
In the present study we tested an algorithm that incorporated the D-index, serial s-GMI and chest, and sinuses CT in high-risk neutropenic patients. We aimed to define a risk stratification parameter to guide a comprehensive diagnostic approach, helping the decision to start antifungal therapy in high-risk neutropenic patients.
Patients and methodsThe study was conducted at the Hospital Universitário Clementino Fraga Filho, Federal University of Rio de Janeiro, Brazil, a tertiary care hospital with ∼400 beds, including a hematology and hematopoietic cell transplant (HCT) unit with eight single-bed rooms with high efficiency particulate air (HEPA) filter and positive pressure, and five double-bed rooms without HEPA filter. The research was conducted in accordance with the Declaration of Helsinki and national and institutional standards. The institution's Ethical Committee (“Comitê de Ética em Pesquisa do Hospital Universitário Clementino Fraga Filho”) approved the study (171/09). The study was registered in ClinicalTrials.gov (NCT00982540).
Between May 2010 and August 2012, all adult patients (age ≥18 years) with AML, acute lymphoid leukemia (ALL) or myelodysplasia (MDS) undergoing induction remission chemotherapy who signed an informed consent were included in the study. We excluded patients with a past history of or an active IMD. Patients were treated in rooms with HEPA filters and received standard care for neutropenia, consisting of antibacterial (ciprofloxacin) and antifungal prophylaxis (usually fluconazole). In case of fever (>38°C), blood cultures were obtained and empiric antibiotic therapy with a β-lactam was started. Blood cultures were repeated in case of persistent or recurrent fever, or as clinically indicated. Modifications in the empirical antibiotic regimen were performed according to the results of cultures and the clinical course of the patient.
Patients were monitored from day 1 of chemotherapy until discharge or death with the D-index and s-GMI performed three times per week (Platelia Aspergillus Ag Kit, Bio-Rad, Marnes-la-Coquette, France). The D-index was calculated using the results of absolute neutrophil counts (ANC) performed three times per week, as previously described.11 Briefly, the calculation of the D-index is based on a graph plotting the ANC during the course of neutropenia, and is the area over the neutrophil curve. Clinically it represents the deficit of neutrophils during the episode.
A workup for IMD (chest and sinus CT scan) was triggered in the following situations: persistent (after six days of antibiotics) or recurrent fever, clinical manifestations of IMD (sinuses, pneumonia, skin nodules), or positive s-GMI (≥0.5). Other tests, including bronchoalveolar lavage and biopsy of skin lesions were performed if clinically indicated. The IMDs were classified as proven, probable, or possible, as previously defined.14,15
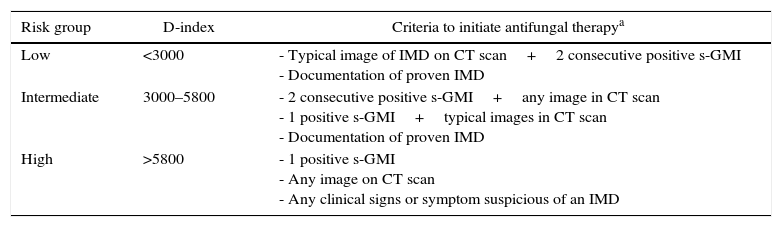
Assuming the cut-off value of 5800 from the original publication11 (negative predictive value of 97%) and 3000 (no documentation of IMD below this value in the retrospective study), we stratified patients in three risk categories based on the cumulative D-index: low (<3000), intermediate (3000–5800), and high (>5800) (Table 1). Patients stratified as high risk and either one positive s-GMI, any image on CT scan or any clinical signs or symptom suspicious of an IMD received systemic antifungal therapy (preferably caspofungin, at the dose of 70mg on day 1 and 50mg on subsequent days, intravenously). Patients in the low risk group received systemic antifungal therapy only in the presence of typical image of IMD on CT scan (well-circumscribed pulmonary infiltrates, air crescent or cavitary lesions) plus two consecutive positive s-GMI, or upon documentation of proven IMD.14 Patients in the intermediate risk group received systemic antifungal therapy in the presence of at least two consecutive positive s-GMI tests plus any image in CT scan, one positive s-GMI in the presence of typical images (well-circumscribed infiltrates, air crescent or cavity) or upon documentation of proven IMD. In case of start of antifungal treatment, the duration of therapy was defined by the attending physician on a case by case basis. Of note, since D-index is a dynamic parameter, patients formerly in the low or intermediate risk were classified as high risk group once the cumulative D-index was >5800.
Risk stratification and criteria to initiate antifungal therapy.
| Risk group | D-index | Criteria to initiate antifungal therapya |
|---|---|---|
| Low | <3000 | - Typical image of IMD on CT scan+2 consecutive positive s-GMI - Documentation of proven IMD |
| Intermediate | 3000–5800 | - 2 consecutive positive s-GMI+any image in CT scan - 1 positive s-GMI+typical images in CT scan - Documentation of proven IMD |
| High | >5800 | - 1 positive s-GMI - Any image on CT scan - Any clinical signs or symptom suspicious of an IMD |
IMD, invasive mold disease; CT, computed tomography; s-GMI, serum galactomannan.
The outcomes of each initial risk group (low, moderate, and high) were compared regarding the incidence of proved and probable IMD, receipt of systemic antifungal therapy and death. Fever was defined as an axillary temperature ≥38°C.
All data were collected in a case report form and analyzed in the HUCFF. Descriptive data were expressed in percentages and medians, with ranges. Dichotomous variables were compared using Chi-square or two-tailed Fisher's exact test. A p-value <0.05 was considered statistically significant. Statistical analyses were performed using SPSS for Windows software (version 15.0.1, SPSS, Inc., USA).
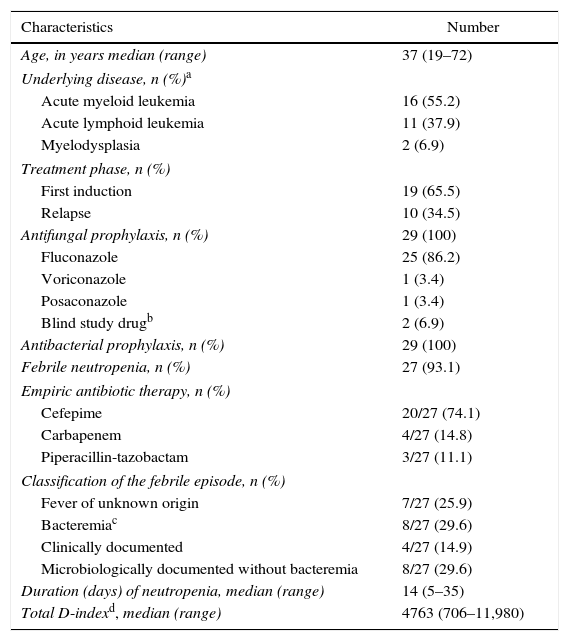
ResultsPatientsDuring the study period a total of 32 episodes of induction remission were assessed for eligibility. Three of these had a previous documentation of IMD, and were excluded. A total of 29 episodes of induction remission in 24 patients (21 males, 87.5%) were included. As shown in Table 2, AML accounted for the majority of episodes (16, 55.2%). All but four patients received fluconazole prophylaxis. Two patients received anti-mold azole agents (voriconazole and posaconazole, 1 each) and two patients received a blind study drug (liposomal amphotericin B or placebo). All patients survived the episode of neutropenia and were discharged.
Characteristics of 29 episodes of induction remission chemotherapy.
| Characteristics | Number |
|---|---|
| Age, in years median (range) | 37 (19–72) |
| Underlying disease, n (%)a | |
| Acute myeloid leukemia | 16 (55.2) |
| Acute lymphoid leukemia | 11 (37.9) |
| Myelodysplasia | 2 (6.9) |
| Treatment phase, n (%) | |
| First induction | 19 (65.5) |
| Relapse | 10 (34.5) |
| Antifungal prophylaxis, n (%) | 29 (100) |
| Fluconazole | 25 (86.2) |
| Voriconazole | 1 (3.4) |
| Posaconazole | 1 (3.4) |
| Blind study drugb | 2 (6.9) |
| Antibacterial prophylaxis, n (%) | 29 (100) |
| Febrile neutropenia, n (%) | 27 (93.1) |
| Empiric antibiotic therapy, n (%) | |
| Cefepime | 20/27 (74.1) |
| Carbapenem | 4/27 (14.8) |
| Piperacillin-tazobactam | 3/27 (11.1) |
| Classification of the febrile episode, n (%) | |
| Fever of unknown origin | 7/27 (25.9) |
| Bacteremiac | 8/27 (29.6) |
| Clinically documented | 4/27 (14.9) |
| Microbiologically documented without bacteremia | 8/27 (29.6) |
| Duration (days) of neutropenia, median (range) | 14 (5–35) |
| Total D-indexd, median (range) | 4763 (706–11,980) |
Chemotherapy regimen – AML: “7+3″ cytarabine+daunorubicin (8), “Flag-dauno” fludarabine, cytarabine, filgastrim and daunorubicin (4), cytarabine, daunorubicin and etoposide (2), decitabine (1); ALL: “Hyper-C-VAD” cyclophosphamide, vincristine, doxorubicin, dexamethasone, cytarabine and methotrexate (8) FLAG-dauno (3); MDS: FLAG-dauno (1), 7+3 (1).
During the study, proven or probable IMD was diagnosed in nine episodes (31%): seven cases of invasive aspergillosis and two of invasive fusariosis. In one episode a diagnosis of possible invasive aspergillosis was made.
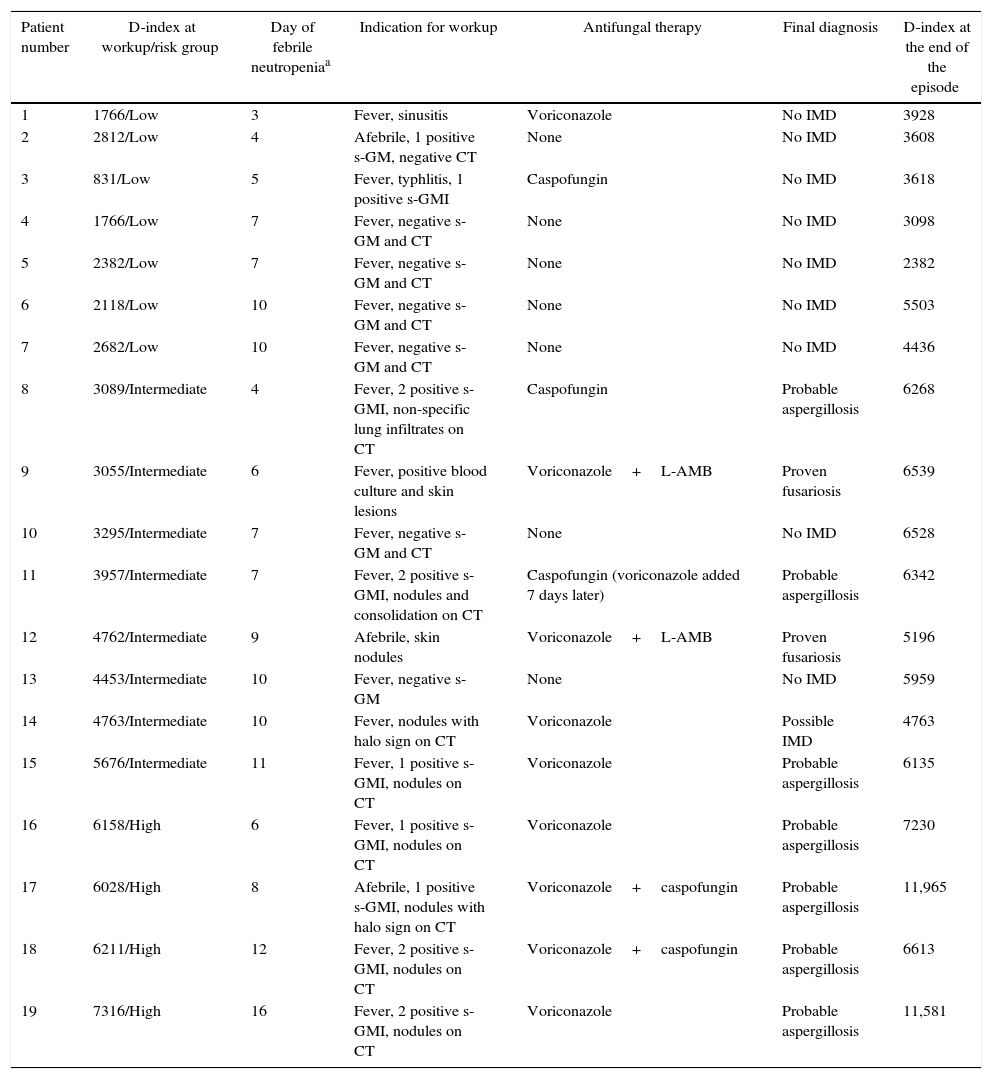
Risk stratification and work-up resultsTwo patients did not develop fever during the episode of neutropenia; one was classified as low-risk as per the final D-index value, and one as high-risk. Among the 27 episodes with fever, eight had no criteria to start an IMD workup, and no antifungal therapy was given: four were classified as low-risk, three were in the intermediate risk, and one was in the high-risk. A workup for IMD was performed in the remaining 19 episodes; antifungal therapy was given in 12 (41.3% of the 29 episodes). Table 3 details the indications of workup for IMD, risk stratification at the time of workup and antifungal therapy in these 19 episodes. Seven episodes were classified as low risk at the moment of workup. While antifungal therapy was given in two, no IMD was diagnosed. Among eight episodes classified as intermediate risk, six received antifungal therapy; proven or probable IMD was diagnosed in five and possible IMD in one. Of note, fever was not present at the time of workup in one of these episodes. The remaining four episodes were classified as high-risk. All received antifungal therapy and had a diagnosis of IMD (all probable invasive aspergillosis).
Workup for invasive mold disease, risk stratification and antifungal therapy in these 19 episodes of febrile neutropenia.
| Patient number | D-index at workup/risk group | Day of febrile neutropeniaa | Indication for workup | Antifungal therapy | Final diagnosis | D-index at the end of the episode |
|---|---|---|---|---|---|---|
| 1 | 1766/Low | 3 | Fever, sinusitis | Voriconazole | No IMD | 3928 |
| 2 | 2812/Low | 4 | Afebrile, 1 positive s-GM, negative CT | None | No IMD | 3608 |
| 3 | 831/Low | 5 | Fever, typhlitis, 1 positive s-GMI | Caspofungin | No IMD | 3618 |
| 4 | 1766/Low | 7 | Fever, negative s-GM and CT | None | No IMD | 3098 |
| 5 | 2382/Low | 7 | Fever, negative s-GM and CT | None | No IMD | 2382 |
| 6 | 2118/Low | 10 | Fever, negative s-GM and CT | None | No IMD | 5503 |
| 7 | 2682/Low | 10 | Fever, negative s-GM and CT | None | No IMD | 4436 |
| 8 | 3089/Intermediate | 4 | Fever, 2 positive s-GMI, non-specific lung infiltrates on CT | Caspofungin | Probable aspergillosis | 6268 |
| 9 | 3055/Intermediate | 6 | Fever, positive blood culture and skin lesions | Voriconazole+L-AMB | Proven fusariosis | 6539 |
| 10 | 3295/Intermediate | 7 | Fever, negative s-GM and CT | None | No IMD | 6528 |
| 11 | 3957/Intermediate | 7 | Fever, 2 positive s-GMI, nodules and consolidation on CT | Caspofungin (voriconazole added 7 days later) | Probable aspergillosis | 6342 |
| 12 | 4762/Intermediate | 9 | Afebrile, skin nodules | Voriconazole+L-AMB | Proven fusariosis | 5196 |
| 13 | 4453/Intermediate | 10 | Fever, negative s-GM | None | No IMD | 5959 |
| 14 | 4763/Intermediate | 10 | Fever, nodules with halo sign on CT | Voriconazole | Possible IMD | 4763 |
| 15 | 5676/Intermediate | 11 | Fever, 1 positive s-GMI, nodules on CT | Voriconazole | Probable aspergillosis | 6135 |
| 16 | 6158/High | 6 | Fever, 1 positive s-GMI, nodules on CT | Voriconazole | Probable aspergillosis | 7230 |
| 17 | 6028/High | 8 | Afebrile, 1 positive s-GMI, nodules with halo sign on CT | Voriconazole+caspofungin | Probable aspergillosis | 11,965 |
| 18 | 6211/High | 12 | Fever, 2 positive s-GMI, nodules on CT | Voriconazole+caspofungin | Probable aspergillosis | 6613 |
| 19 | 7316/High | 16 | Fever, 2 positive s-GMI, nodules on CT | Voriconazole | Probable aspergillosis | 11,581 |
IMD, invasive mold disease; s-GMI, serum Aspergillus galactomannan antigen; CT, computed tomography; L-AMB, lipid amphotericin B.
Overall, considering febrile and afebrile patients, workup for IMD was necessary in 4 of 6 (66.7%) episodes classified as high-risk, 8 of 11 (72.7%) classified as intermediate risk, and 7 of 12 (58.3%) classified as low risk (p=0.77). Antifungal therapy was given to 4 of 6 (66.7%) episodes classified as high-risk, 6 of 11 (54.5%) classified as intermediate risk, and 2 of 12 (16.7%) classified as low-risk group (p=0.07). Finally, proven or probable IMD was diagnosed in 4 of 6 (66.7%) episodes classified as high risk, 5 of 11 (45.5%) classified as intermediate risk, and in none of the 12 episodes classified as low risk (p=0.007).
A total of 299 s-GMI tests were performed, with a median of 10 tests per episode (range 2–27). Of those, 51 tests (17.0%) were positive in 20 episodes (68.9% of the episodes). The test was considered false positive in 11 of the 20 episodes based on clinical judgment. In seven of these 11 episodes the patient was classified as low risk at the time of workup (or at the end of the episode if workup was not undertaken); the other four episodes were classified as intermediate or high risk (3 and 1, respectively). In these four episodes, only one s-GMI test was positive among 8, 9, 11, and 16 tests performed per episode, respectively. In the low-risk group, all seven positive s-GMI tests were false positive, compared to three out of eight (37.5%) in the intermediate risk group and one out of five (20%) in the high risk group (p=0.01).
DiscussionIn this study we showed that a dynamic assessment of the risk of IMD using the D-index may be of help to decide whether antifungal therapy should be given to neutropenic patients. Using this strategy that incorporated the D-index and serial s-GMI assessment we were able to avoid the initiation of antifungal therapy based on just the presence of fever, and to start an antifungal agent in afebrile patients, or earlier than day 7 of empiric antibiotic therapy in patients at high risk who presented an indirect sign of IMD.
Empiric antifungal therapy consists in the initiation of a systemic antifungal agent in neutropenic patients who persist febrile on day 4–7 of antibiotic therapy or who present recurrence of fever.16 Although its basis lacks solid evidence,17,18 it became standard of care and is still endorsed by guidelines.19,20 However, since the empiric therapy strategy uses a non-specific parameter (fever) to trigger the initiation of antifungal therapy, many patients receive antifungal therapy unnecessarily. In contrast with the empiric strategy, a diagnostic-driven (or pre-emptive) approach takes into account other parameters (such as positive s-GMI, PCR, images and others) to initiate antifungal therapy. One randomized and some non-randomized studies showed that the diagnostic-driven approach can replace the empiric strategy provided that the center is able to provide these tools in a timely fashion.5–7,19–22
In the present study we tested an algorithm for the decision to start antifungal therapy that incorporates the D-index. This tool is inexpensive and easy to calculate. Conceptually the D-index measures the deficit of neutrophils, taking into account both the severity and the duration of neutropenia. A spread sheet is used to calculate the area over the neutrophil curve, plotting the results of the ANC over the course of neutropenia. At each new ANC value, a cumulative D-index is calculated. The tool was retrospectively evaluated in a small group of patients with IMD, and for a cut-off value of 5800 the NPV was very high.11
In the present study we selected a population of patients at high risk for IMD: acute leukemia or MDS undergoing induction remission chemotherapy. Indeed, nine episodes of proven or probable IMD (31%) and one of possible IMD were diagnosed. Caspofungin was chosen as the antifungal agent in patients with suspicion of IMD because of its indication and large use as empiric therapy in neutropenic patients,23 and because of its favorable safety profile. Considering that antifungal therapy was given in 12 episodes, only two patients received antifungal therapy unnecessarily. In addition, the application of the algorithm allowed us to start antifungal therapy earlier than would be expected if the empiric approach had been used: by day 7 of empiric antibiotic therapy five patients were already on antifungal therapy.
A cornerstone of the diagnostic driven approach is s-GMI. Two different strategies have been evaluated in different studies. In one strategy serial s-GMI testing is performed during the whole period at risk.6 In the other strategy, s-GMI is obtained daily when patients are at high risk for IMD and present persistent or recurrent fever or any sign suggestive of an IMD.22 The two strategies have advantages and disadvantages. A potential disadvantage of the first strategy is false positive s-GMI. In addition to the potential interference of the test by the use of anti-mold antifungal agents,24 the pre-test probability of IMD is an important parameter in evaluating the PPV of the test; the higher the pre-test probability of the disease the higher is the PPV.25 Since the risk of invasive aspergillosis is a time function of the duration of neutropenia,26 the D-index can be of help in the interpretation of the performance of serum biomarkers. With a dynamic risk stratification based on the intensity and duration of neutropenia, we can estimate the pre-test probability of IMD looking at the risk group. Indeed, none of the patients considered as low risk at the time of workup for IMD who had positive s-GMI had a diagnosis of IMD. These data suggest that we can use the D-index risk stratification to estimate the pre-test and post-test probability of s-GMI.
The performance of the risk stratification based on the D-index can be appreciated by looking at the incidence of IMD in the three risk categories. Furthermore, this strategy resulted in a potential comprehensive use of antifungals if we compare with the classical empiric antifungal strategy, and antifungal therapy was started in two afebrile patients, both with IMD. Finally, the appropriate timing of initiation of antifungal therapy may have been critical to the favorable outcome of patients with IMD in this cohort.
Our study has an important limitation, the small sample size. Therefore, future studies with a larger number of patients are needed. Nevertheless, the results of our study may have important clinical implications. Our data suggest that the incorporation of the D-index in an algorithm of diagnostic-driven antifungal therapy may improve the performance of fungal biomarkers, including s-GMI. With this regard, and considering that patients in the low-risk group undertaking workup for IMD are very likely to have false negative s-GMI, clinicians may wait for more data (e.g. other positive s-GMI) in order to trigger additional tests or to initiate antifungal therapy. Alternatively, clinicians may start monitoring with s-GMI only once the cumulative D-index is above 3000. By contrast, given the higher proportion of true positive s-GMI in patients classified as intermediate or high risk by D-index, additional workup and/or prompt initiation of antifungal therapy may be a better option if s-GMI test is positive.
ConclusionsIn conclusion, the risk stratification using the D-index was a useful tool to a more comprehensive antifungal strategy, resulting in reduction of empirical therapy in low risk patients, and guiding an earlier start of antifungal agents in afebrile patients at high risk who presented an indirect sign of IMD. Future studies with larger sample size are needed.
FundingThis work was supported Merck, and Conselho Nacional de Desenvolvimento Científico e Tecnologico – CNPq (PDJ – 159794/2011-0 to MG, and 305291/2013-0 and 474555/2013-5 to MN).
AuthorshipMG, RP and MN conceived and designed the experiments; MG, AS and LM performed the experiments; MG and MN analyzed the data; MG and MN contributed analysis tools; MG, RP and MN contributed to the writing of the manuscript; MG, AS, LM, RP and MN revised and approved the manuscript.
Conflicts of interestThis work was supported Merck. M.N. serves on scientific advisory boards for Pfizer, Merck, Astellas Pharma Inc., Gilead Sciences and Basilea, and has received speaker honoraria from Pfizer, Inc., Merck, Astellas Pharma and Gilead Sciences. All other authors declare no conflicts of interest.
Register number in ClinicalTrials.gov: NCT00982540.