Bathing with 2% chlorhexidine (CHG) wipes is an important measure regarding infection prevention in critically ill patients. The aim of this study was to evaluate the impact of CHG wipes bath to prevent central-line associated bloodstream infection (CLABSI) in critically ill patients and determine if such measure is cost-saving.
Methodsa quasi-experimental study, conducted from July 2017 to April 2019. Daily bath with 2% CHG was used in all patients at the unit in the intervention period. The following were evaluated: CLABSI incidence density in both periods, 30- day mortality, guided antimicrobials used to treat CLABSI and 2% CHG costs.
ResultsCLABSI incidence density dropped from 8.69 to 1.83 per 1.000 central line-days (p = 0.001), mainly by Klebsiella pneumoniae Carbapenen Resistant (Kp-KPC) (p = 0.05). Costs with guided antimicrobials for the treatment in pre-intervention were US$ 46,114.36, and in the intervention period, US$ 4,177.50. The 2% CHG monthly cost was US$ 2,698.00, achieving 30% savings when comparing both periods.
DiscussionAn expressive reduction of 79% in CLABSI incidence density was observed, mainly due to Kp-KPC infection and also a reduction in guided antimicrobial costs.
ConclusionsBathing with 2% CHG led to evident CLABSI reduction.
Central-line associated bloodstream infection (CLABSI) is one of the main causes of healthcare-associated infections (HAI) in Intensive Care Units (ICU), contributing to prolonged hospitalization periods and increased morbimortality.1–4
The incidence of CLABSI in ICU may vary from 1.7 to 44.6 per 1.000 central line-days.1,2 Studies indicate an attributed mortality of CLABSI to be 2.27–2.75%,3,4 reaching up to 34% if the infection is caused by carbapenem resistant Klebsiella pneumoniae.5
Several studies have shown that bathing with 2% chlorhexidine (CHG) wipes is an important preventive measure of infection in critically ill patients, mainly of CLABSI.6–11 Previous studies also support the use of CHG bathing as a cost-saving practice. Reagan et al. demonstrated by mathematical models how the effectiveness of CHG bathing impacts the overall return of health care savings.12
However, there have been report showing that bathing with CHG is effective in reducing infection by Gram positive cocci but there is still no consensus regarding Gram negative bacilli.13
Costs with antibiotics for CLABSI treatment may vary from US$ 33,000 to 41,000,3,12,14 being especially higher if the CLABSI is caused by multiresistant microorganisms, which are most incident in ICU patients.
At our clinical ICU, CLABSI infection was higher than expected (8.69 per 1000 central line-days). Despite the classic measures for CLABSI prevention, we did not manage to reduce CLABSI incidence density. As an additional measure of infection control, we introduced daily bathing with 2% CHG wipes, considering our previous experience in reducing CLABSI and carbapenem-resistant Enterobacteriacea at the surgical ICU.11
ObjectivesTo evaluate the impact of daily bath with 2% CHG in preventing CLABSI in critically ill patients.
To evaluate the cost savings of daily bath with 2% CHG in preventing CLABSI in critically ill patients.
MethodsA quasi-experimental study was conducted to evaluate the impact of 2% CHG daily bath at the clinical ICU of our institution.
Hospital and patientsThe study was undertaken in a 374-bed tertiary public cardiovascular surgery teaching hospital that performs approximately 2000 cardiac procedures per year. The intervention took place in the clinical ICU, which has a total capacity of 10 beds. In this ICU are hospitalized patients with chronic heart disease and multiple comorbidities.
All patients were routinely submitted to surveillance culture collection for institution of contact precautions in positive cases of MDR, on admission and weekly. The colonization pressure was calculated as the ratio of the number of new colonization during the period and the number of patient-days multiplied by 100.
The hospital infection control service performs active surveillance following the National Healthcare Safety Network recommendations.15 Surveillance for healthcare-associated infections (HAIs) including CLABSI follows the criteria established by the US Centers for Disease Control and Prevention (CDC).16
Study designThis quasi-experimental study was divided into two periods: (i) pre-intervention period (July 2017 to May 2018); and (ii) intervention period (June 2018 to Apr 2019). The following data were evaluated: CLABSI, catheter-associated urinary tract infection (CA-UTI) and ventilator associated pneumonia (VAP) incidence density rates in both periods, 30- day mortality for CLABSI, Defined Daily Dose (DDD), Days of Therapy (DOT) and Length of Therapy (LOT) of guided antimicrobials used to treat CLABSI in both periods and the monthly cost of 2% CHG wipes at the intervention period.
InterventionDaily bath with 2% CHG wipes was introduced at the intervention period to all patients hospitalized at the ICU, from hospital admittance to discharge or death. The nursing staff received training from the manufacturer on how to apply the wipes.
The price of the 2% CHG was established according to a public bidding process.
No additional measures have been implemented, continuing education about infection prevention and antimicrobial stewardship activities have not changed in the two periods.
AntibioticsFor this study, costs with guided antibiotics were considered, that is, antibiotics prescribed after the microorganism detection.17,18 Empiric antimicrobial therapies initiated when the symptoms of bacteremia or sepsis began were considered for the calculation only for unity DDD. After detection of the microorganism at blood cultures, the infectious diseases (ID) doctors based their procedures on the stewardship program of the institution and escalated or deescalated the antimicrobial therapy. Prices of antibiotics were obtained from the Drug Market Regulation Chamber (CMED) at the Brazilian Health Surveillance Agency (ANVISA).19
Microbiological analysisAccording to international terminology created by the European Centre for Disease Control (ECDC) and US CDC, Atlanta, multidrug-resistant (MDR) microorganisms were so defined if they were not susceptibility to at least one agent in three or more antimicrobial categories.20
All bacterial isolates were identified by mass spectrometry using the MS Vitek system (bioMerieux, Marcy-l’Etoile, France). Susceptibility tests were performed using the Vitek 2 system (bioMerieux) and resistance profiles were defined according to M100-S25 of the Clinical and Laboratory Standards Institute (CLSI).21 The screening test of carbapenemase was performed using the modified Hodge test, as recommended by CLSI. Detection of carbapenemase (blaKPC,blaNDM,blaIMP, blaVIM, blaGES and blaOXA-48-like) genes was determined by real-time polymerase chain reaction (RT-PCR).21,22
Statistical analysesDifferences in proportions were compared using the Fisher’s exact test, and p < 0.05 was considered to indicate statistical significance.
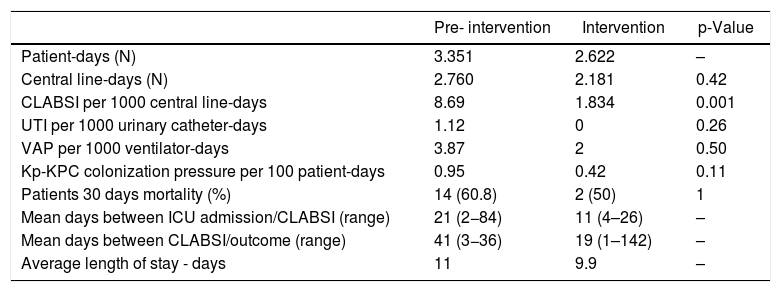
ResultsThe pre-intervention period involved 2760 central line-days and 24 CLABSI events; the intervention period involved 2181 central line-days and four CLABSI events. The incidence density rate dropped from 8.69 to 1.83 per 1.000 central line-days (p = 0.011), as shown in Table 1. Incidence density rates of CA-UTI and VAP showed a decrease but not statistically significant. There was a reduction in length of stay when comparing the two periods (11 to 9.9 days).
Intensive care unit epidemiological characteristics - pre and during intervention periods.
| Pre- intervention | Intervention | p-Value | |
|---|---|---|---|
| Patient-days (N) | 3.351 | 2.622 | – |
| Central line-days (N) | 2.760 | 2.181 | 0.42 |
| CLABSI per 1000 central line-days | 8.69 | 1.834 | 0.001 |
| UTI per 1000 urinary catheter-days | 1.12 | 0 | 0.26 |
| VAP per 1000 ventilator-days | 3.87 | 2 | 0.50 |
| Kp-KPC colonization pressure per 100 patient-days | 0.95 | 0.42 | 0.11 |
| Patients 30 days mortality (%) | 14 (60.8) | 2 (50) | 1 |
| Mean days between ICU admission/CLABSI (range) | 21 (2−84) | 11 (4–26) | – |
| Mean days between CLABSI/outcome (range) | 41 (3−36) | 19 (1–142) | – |
| Average length of stay - days | 11 | 9.9 | – |
Note: CLABSI: central line-associated bloodstream infections; Kp-KPC (Klebsiella pneumoniae KPC producer); UTI: urinary tract infection; VAP: ventilator associated pneumonia.
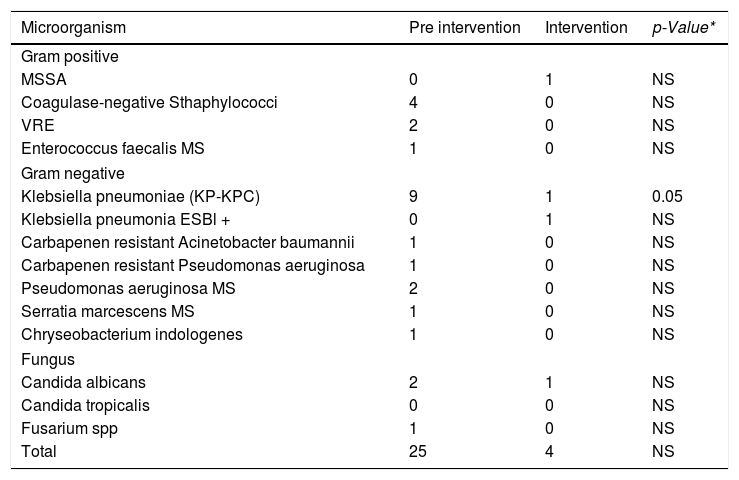
Among the 24 episodes of CLABSI in the pre-intervention period, 11 different microorganisms were isolated, 62% (15/24) were Gram-negative bacilli, out of these, nine were Kp-KPC, 29% (7/24) were Gram-positive cocci and 12% (3/24) fungi. Fifty-four percent of the cases were caused by MDR bacteria (Table 2).
CLABSI causative microorganisms distribution in pre and during intervention periods.
| Microorganism | Pre intervention | Intervention | p-Value* |
|---|---|---|---|
| Gram positive | |||
| MSSA | 0 | 1 | NS |
| Coagulase-negative Sthaphylococci | 4 | 0 | NS |
| VRE | 2 | 0 | NS |
| Enterococcus faecalis MS | 1 | 0 | NS |
| Gram negative | |||
| Klebsiella pneumoniae (KP-KPC) | 9 | 1 | 0.05 |
| Klebsiella pneumonia ESBl + | 0 | 1 | NS |
| Carbapenen resistant Acinetobacter baumannii | 1 | 0 | NS |
| Carbapenen resistant Pseudomonas aeruginosa | 1 | 0 | NS |
| Pseudomonas aeruginosa MS | 2 | 0 | NS |
| Serratia marcescens MS | 1 | 0 | NS |
| Chryseobacterium indologenes | 1 | 0 | NS |
| Fungus | |||
| Candida albicans | 2 | 1 | NS |
| Candida tropicalis | 0 | 0 | NS |
| Fusarium spp | 1 | 0 | NS |
| Total | 25 | 4 | NS |
Note: Fisher exact test- p < 0,05; MS: Multi sensitive; NS: Not Significant; VRE Vancomycin Resistant Enterococcus spp.
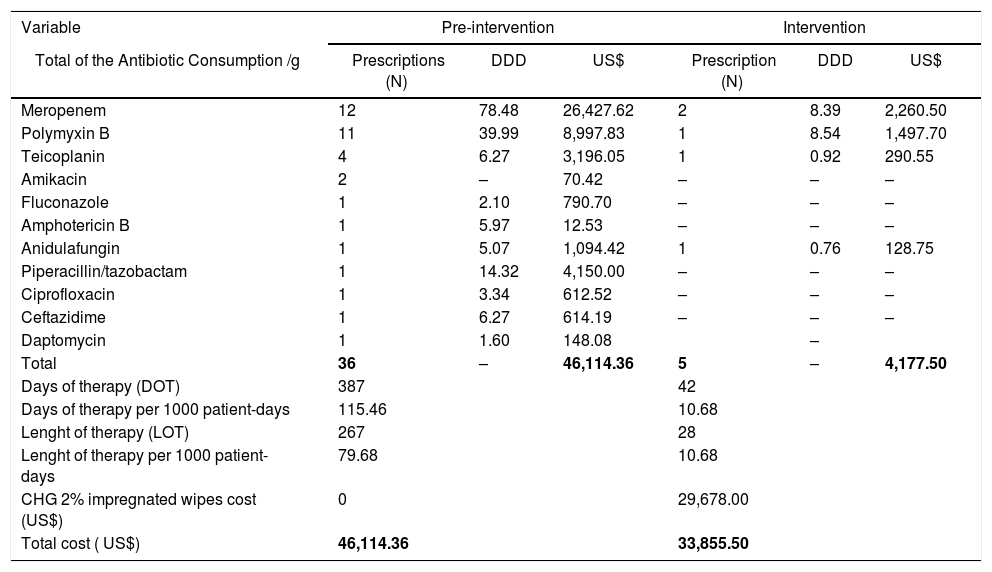
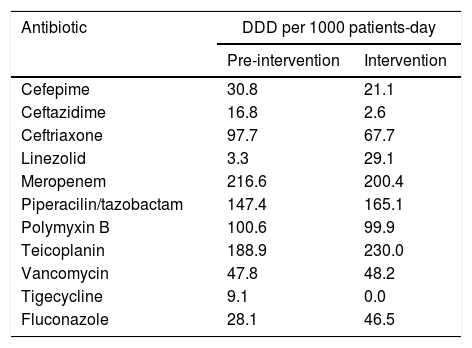
The most prescribed antibiotics in both periods were meropenem (14 prescriptions) and polymyxin B (12 prescriptions), considering that the combination of these two antibiotics are the preferred option for Kp-KPC treatment in our hospital routine. Antimicrobial-use time ranged from two to 42 days, with an average of 9.9 days and a median of nine days. The DDD, DOT and LOT per 1000 patient-days are shown in Table 3. A reduction in guided antimicrobial usage was observed during the intervention period; however, there was no difference in the consumption of the main antimicrobials (unity DDD) when considered empirical and guided treatments between the two periods (Table 4).
CLABSI guided antimicrobial consumption and costs with antibiotics and CHG wipes - Pre and during intervention periods.
| Variable | Pre-intervention | Intervention | ||||
|---|---|---|---|---|---|---|
| Total of the Antibiotic Consumption /g | Prescriptions (N) | DDD | US$ | Prescription (N) | DDD | US$ |
| Meropenem | 12 | 78.48 | 26,427.62 | 2 | 8.39 | 2,260.50 |
| Polymyxin B | 11 | 39.99 | 8,997.83 | 1 | 8.54 | 1,497.70 |
| Teicoplanin | 4 | 6.27 | 3,196.05 | 1 | 0.92 | 290.55 |
| Amikacin | 2 | – | 70.42 | – | – | – |
| Fluconazole | 1 | 2.10 | 790.70 | – | – | – |
| Amphotericin B | 1 | 5.97 | 12.53 | – | – | – |
| Anidulafungin | 1 | 5.07 | 1,094.42 | 1 | 0.76 | 128.75 |
| Piperacillin/tazobactam | 1 | 14.32 | 4,150.00 | – | – | – |
| Ciprofloxacin | 1 | 3.34 | 612.52 | – | – | – |
| Ceftazidime | 1 | 6.27 | 614.19 | – | – | – |
| Daptomycin | 1 | 1.60 | 148.08 | – | ||
| Total | 36 | – | 46,114.36 | 5 | – | 4,177.50 |
| Days of therapy (DOT) | 387 | 42 | ||||
| Days of therapy per 1000 patient-days | 115.46 | 10.68 | ||||
| Lenght of therapy (LOT) | 267 | 28 | ||||
| Lenght of therapy per 1000 patient-days | 79.68 | 10.68 | ||||
| CHG 2% impregnated wipes cost (US$) | 0 | 29,678.00 | ||||
| Total cost ( US$) | 46,114.36 | 33,855.50 | ||||
Note: DDD Defining Daily Doses; all KPC CLABSI patients were treated with combination therapy. Cost US$ American dollars - conversion value 1 dollar = 3.87 reais (2018).
Unity antimicrobial consumption by DDD per 1000 patients-days – pre and during intervention periods.
| Antibiotic | DDD per 1000 patients-day | |
|---|---|---|
| Pre-intervention | Intervention | |
| Cefepime | 30.8 | 21.1 |
| Ceftazidime | 16.8 | 2.6 |
| Ceftriaxone | 97.7 | 67.7 |
| Linezolid | 3.3 | 29.1 |
| Meropenem | 216.6 | 200.4 |
| Piperacilin/tazobactam | 147.4 | 165.1 |
| Polymyxin B | 100.6 | 99.9 |
| Teicoplanin | 188.9 | 230.0 |
| Vancomycin | 47.8 | 48.2 |
| Tigecycline | 9.1 | 0.0 |
| Fluconazole | 28.1 | 46.5 |
The cost with guided antimicrobials for CLABSI treatment in the pre-intervention period was US$ 46,114.36 and 4,177.50 in the intervention period (Table 3).
In the intervention period, the average monthly consumption of CHG was 190 towels per month, with unit value of US$ 14.20, generating a monthly cost of US$ 2,698.00. Considering costs with guided antimicrobials in pre-intervention period and total costs (CHG wipes plus guided antimicrobials) in intervention period, there was a saving of 12,000 US$ dollars.
The colonization pressure by Kp-KPC in both periods are shown in Table 1, it is possible to verify a reduction of more than 50% (p = 0.11).
DiscussionOur clinical ICU is addressed to patients with heart diseases followed at our institution. In the intervention period, we noticed a decrease in the number of patient-days and central line-days, explained by a reduction in number of ICU beds due to administrative measures, but without changing in nurse-to-patient ratio or inpatient characteristics.
In this quasi-experimental study, there was a significant reduction in the intervention period not only in CLABSI, but also in CLABSI by Kp-KPC, which was the most prevalent infection in our ICU. A hypothetical explanation is that with the reduction of colonization pressure there was a decrease in cross-transmission.23,24
Although controversial, some studies demonstrated that a reduction in KPC colonization was achieved with 2% CHG bath.25–27 Lin et al. demonstrated a significant reduction in skin colonization after this practice25 and Muñoz-Price in a quasi-experimental study in a long term acute care hospital also showed a significant decrease in KPC colonization and clinical cases.26
Another interesting data in our work is about antimicrobial consumption. We demonstrated that there was a reduction in CLABSI guided antimicrobial consumption, but, we did not find a reduction in unity DDD in both periods. Although DDD is not a good indicator of antimicrobial consumption and LOT was not available for analysis, an explanation for this findings is that no expressive reduction in VAP and CA-UTI could be demonstrated. In addition, many ICU patients had chronic clinical conditions and multiple comorbidities, leading the medical team to introduce empiric antimicrobial therapy if when clinically indicated. However, we believe that it may also happen in real life in some ICUs.
When considering costs, we observed considerable savings in CLABSI guided therapy. Combined with a reduction in CLABSI rates of patients during the intervention period, we hypothesize that there were savings for the entire unity, but more specific pharmacoeconomic studies should be conducted to answer this question.
Due to scarce therapeutic options for MDR bacteria treatment, investment in new technologies aiming HAI reduction is essential. The tested intervention in our institution was very beneficial and has already been incorporated into our routine as a preventive measure of CLABSI and decolonization of MDR bacteria.
Regardless, more studies should be carried out to evaluate the impact of CHG 2% bath on MDR colonization and on infections by the same agent in critically ill patients.
As limitations of this study, it was conducted at a single ICU of a cardiovascular reference hospital, the clinical characteristics of included ICU patients were not evaluated, and reduction in the unity DDD could not be demonstrated during the intervention period.
Other important limitation of quasi-experimental studies in general is the influence of Hawthorne effect, since the interventions were not blinded.
ConclusionAt our ICU, the introduction of daily bath with 2% CHG wipes led to an evident reduction in CLABSI and in MDR bacterial infection, mainly Kp-KPC. This intervention can be a useful in ICU with a high CLABSI rates, in addition to being economically advantageous.
Conflicts of interestThe author declares no conflicts of interest.