Homeless people are at high risk for sexually transmitted infections (STIs), such as human immunodeficiency virus (HIV) infection and syphilis. We investigated the epidemiology of HIV-1 infection and syphilis among homeless individuals in a large city in Central-Western Brazil. In this cross-sectional study, we interviewed and tested 355 individuals from September 2014 to August 2015. Rapid test samples positive for syphilis were retested using the Venereal Disease Research Laboratory (VDRL) test. Blood samples from HIV-infected participants were collected for POL sequencing using HIV-1 RNA extracted from plasma, reverse transcription, and nested polymerase chain reaction. Anti-HIV-1-positive samples were subtyped by sequencing the nucleotides of HIV-1 protease and part of the HIV-1 reverse transcriptase genes. Transmitted and acquired drug resistance mutations and susceptibility to antiretroviral drugs were also analyzed. Anti-HIV was positive in 14 patients (3.9%; 95% confidence interval [CI]: 2.3–6.4). HIV-1 RNA was detected in 8 of the 14 samples. Two of the eight (25%) isolates showed HIV-1 drug resistance mutations. Furthermore, 78 (22%; 95% CI: 17.9–26.5) and 29 (8.2%; 95% CI: 5.6–11.4) homeless individuals tested positive for syphilis using the rapid test and VDRL test, respectively. Two individuals were anti-HIV-1 and VDRL test positive. Daily alcohol use (adjusted odds ratio [AOR]: 3.2, 95% CI: 1.0–10.4), sex with people living with HIV (PLWH) infection (AOR: 6.8, 95% CI: 1.9–25.0), and sex with people of the same sex (AOR: 5.4, 95% CI: 1.7–17.5) were predictors of HIV infection. Age ≤35 years (AOR: 3.8, 95% CI: 1.4–10.8), previous syphilis testing (AOR: 3.5, 95% CI: 1.4–8.4), history of genital lesions (AOR: 4.9, 95% CI: 1.3–19.1), and crack use in the last six months (AOR: 3.1, 95% CI: 1.3–7.6) were predictors of syphilis. Our findings highlight the importance of STI prevention and control strategies among the homeless.
According to the Institute of Global Homelessness, homeless people are defined as those who live in severely inadequate housing due to a lack of access to minimally adequate housing conditions.1 In general, this population has low educational levels, poor hygiene conditions, low income, high rates of unemployment, poor nutrition, and limited access to healthcare services.2 The consumption of alcohol and illicit drugs, as well as prostitution, are common behaviors among those who live on the streets.3 These vulnerabilities contribute to an elevated risk of sexually transmitted infections (STIs) in this population, such as human immunodeficiency virus (HIV) infection and syphilis.4
Globally, it is estimated that 37.9 million (95% confidence interval [CI]: 32.7–44.0) people are living with HIV infection.5 Despite a substantial decrease in the incidence of infection in recent decades (2.9 million in 1997 to 1.7 million in 2018) because of the availability of wide antiretroviral (ARV) treatments,5 the upsurge of HIV-1 isolates with mutations associated with drug resistance has been a large challenge for healthcare providers and researchers.6
The emergence of HIV infection in the 1980s contributed to the global increase of other STIs such as syphilis.7 This infection, which is caused by Treponema pallidum, has resulted in substantial morbidity and mortality.8 In 2016, the number of individuals with syphilis worldwide was estimated to be 19.9 million, and approximately 6.3 million new cases were observed in the age group of 15–49 years.9 Individuals with syphilis are 2–5 times more likely to transmit or acquire HIV infection than those who are not infected with a STI.10
Worldwide, the number of people without a place to live is roughly estimated to be 3.8–216 million, with 33.6-179 million living on the streets.11 In Brazil, the number of people without a place to live is estimated to be 102,000,12 and 8777 live in the central-western region, which includes the states of Goiás, Mato Grosso, and Mato Grosso do Sul, and the Federal District including Brasilia.
Homeless individuals access healthcare services preferentially in hospitals and emergency units.4,13,14 Lack of identity documentation, of knowledge about where and how to obtain assistance, of a fixed address, long waiting times for health care, discrimination, fear, and competing priorities for their own subsistence act as barriers to accessing healthcare services.4,14
In Brazil, the HIV-1 infection epidemic is concentrated, mainly affecting individuals who are most socially vulnerable, such as people who live on the streets.15–18 There is little information regarding the epidemiology of STIs in this population, and to the best of our knowledge, no data on the variability of HIV-1 in this population exist. This study aimed to investigate the epidemiology of HIV infection and syphilis, HIV-1 subtypes, and ARV drug resistance among homeless people living in a metropolitan area of Central-Western Brazil, one of the largest cities in this region.
Material and methodsA cross-sectional study was conducted from September 2014 to August 2015 at a public shelter in Goiânia, which is the second largest city in the central-western region of Brazil. It is estimated that there are 0.25 homeless/1000 inhabitants.12 The epidemiology of STIs such as HIV infection and syphilis among the homeless was evaluated.
Individuals aged above 18 years who slept at least one night in the public shelter were included in the study. Those who expressed aggressive behavior that put the research team at risk were excluded.
The sample size estimated for the study was 342 homeless people, considering a statistical power of 80% (β=20%), significance level of 95% (α=0.05), HIV prevalence of 4.9%,16 and design effect correction of 2.0.
As described previously,19 all eligible individuals were interviewed in a private place, using a structured instrument comprising questions about sociodemographic characteristics and predictors of STIs. After the interview, all participants were tested for syphilis, using a rapid immunochromatographic assay (SD BIOLINE Syphilis 3.0, Standard Diagnostics Inc., Korea), and anti-HIV-1/2 using two HIV rapid tests: Rapid Check HIV 1&2 (NDI-UFES, Brazil) and Dual Path Platform (DPP®) HIV 1/2 (Bio-Manguinhos/Fiocruz, Brazil).
Blood samples (5mL) were collected from all participants who were positive for syphilis and/or anti-HIV by rapid tests. Participants positive for syphilis were retested using the Venereal Disease Research Laboratory (VDRL) test (Wiener LabGroup, Rosario, Argentina). Lifetime syphilis was diagnosed when individuals were positive for rapid test (treponemal test). Active syphilis was diagnosed if individuals were positive for rapid test and VDRL (non-treponemal test) test, independent of the VDRL titers.
According to the method described by Cardoso et al.,20 plasma RNA was extracted using the QIAamp Viral RNA Mini Kit (Qiagen GmbH, Hilden, Germany) and retrotranscribed into complementary DNA and entire protease region. Moreover, approximately 750bp of the reverse transcriptase fragment was amplified by nested polymerase chain reaction, and their products were sequenced. HIV-1 subtypes were identified using the REGA genotyping tool version 2.0, and phylogenetic inference was performed using reference sequences obtained from the Los Alamos HIV Database (http://hiv_web.lanl.gov). The phylogenetic tree was constructed by the neighbor joining method under Kimura’s two-parameter correction model using MEGA version 5 software. Bootstrap values (1000 replicates) above 70% were considered significant. The transmitted drug resistance (TDR) rate among ARV-naive patients was determined using the Calibrated Population Resistance tool (Stanford Surveillance Drug Resistance Mutations). Secondary drug resistance mutations and resistance profiles were defined using the Stanford HIV Drug Resistance Database. The ARV mutation susceptibility profile was also analyzed using the Stanford HIV Drug Resistance Database.
All HIV-1 sequences generated in this study were deposited in the GenBank database under accession numbers MK499366-MK499373.
HIV infection and syphilis were the dependent variables. The sociodemographic (sex, age, race, educational level, marital status, and sleep on the street) and other explanatory variables such as daily alcohol consumption, injection drug use, cocaine use, marijuana smoking, and crack cocaine use in the past 6 months, age at first sexual intercourse, previous sexual violence, sexual partners in lifetime, condom use with a steady partner, condom use with a casual partner, exchange sex for money or drugs, sex with sex workers, sex with a drug user, sex with person living with HIV, same-sex sexual activity, anal sex, sharing of objects for personal use, previously incarcerated, tattoos/piercings, previous HIV and syphilis testing, and history of genital lesions were the independent variables.
The data were analyzed using the Statistical Package for the Social Sciences version 17.0. Prevalence was calculated with 95% CIs. Data of the descriptive analysis were expressed as proportions. For the analysis of potential predictors of exposure to HIV infection and active syphilis, the chi-squared test (χ2) or Fisher’s exact test were used to assess the differences between proportions. For the calculation of the adjusted odds ratio (AOR), variables that presented a value of p<0.20 were included in a forward stepwise logistic regression model. For this study, p-values<0.05 were considered statistically significant.
This study was approved by the Ethics Committee of the Federal University of Goiás under protocol number 045/13. Written informed consent was obtained from all participants.
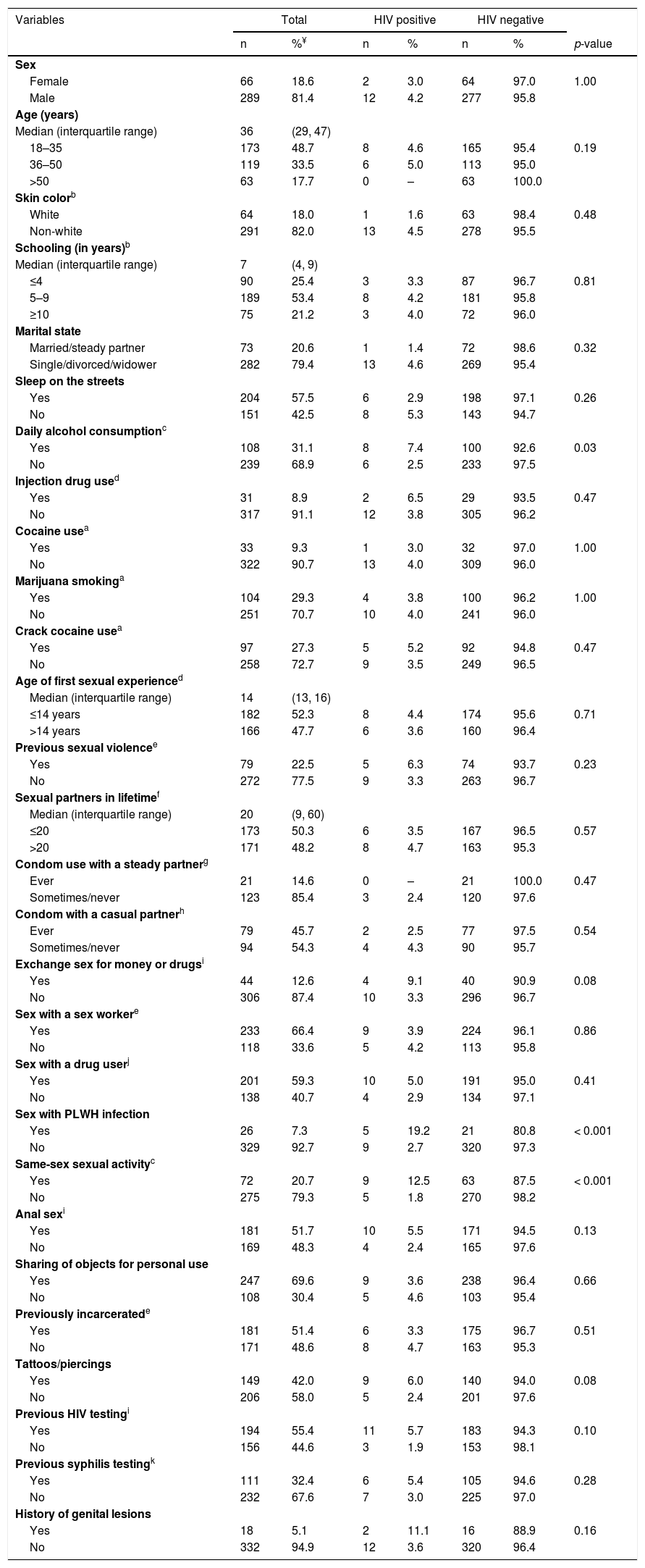
ResultsTables 1 and 2 show the characteristics and univariate analysis of potential factors associated with the prevalence of HIV infection and syphilis in the population studied, respectively. A total of 355 eligible individuals completed the survey. Of these, 81.4% were male, 20.6% were married, median age 36 years (interquartile range [IQR]: 29–47), and majority non-white (82.0%), and with low educational level (78.8% with nine years or less of formal education). Most individuals had experienced sleeping on the streets (57.5%). The median length of stay in the shelter was 10 days (IQR: 4–30).
Univariate analysis of factors associated with the prevalence of human immunodeficiency virus infection among 355 homeless people in Goiânia, Central-Western Brazil.
| Variables | Total | HIV positive | HIV negative | ||||
|---|---|---|---|---|---|---|---|
| n | %¥ | n | % | n | % | p-value | |
| Sex | |||||||
| Female | 66 | 18.6 | 2 | 3.0 | 64 | 97.0 | 1.00 |
| Male | 289 | 81.4 | 12 | 4.2 | 277 | 95.8 | |
| Age (years) | |||||||
| Median (interquartile range) | 36 | (29, 47) | |||||
| 18–35 | 173 | 48.7 | 8 | 4.6 | 165 | 95.4 | 0.19 |
| 36–50 | 119 | 33.5 | 6 | 5.0 | 113 | 95.0 | |
| >50 | 63 | 17.7 | 0 | – | 63 | 100.0 | |
| Skin colorb | |||||||
| White | 64 | 18.0 | 1 | 1.6 | 63 | 98.4 | 0.48 |
| Non-white | 291 | 82.0 | 13 | 4.5 | 278 | 95.5 | |
| Schooling (in years)b | |||||||
| Median (interquartile range) | 7 | (4, 9) | |||||
| ≤4 | 90 | 25.4 | 3 | 3.3 | 87 | 96.7 | 0.81 |
| 5–9 | 189 | 53.4 | 8 | 4.2 | 181 | 95.8 | |
| ≥10 | 75 | 21.2 | 3 | 4.0 | 72 | 96.0 | |
| Marital state | |||||||
| Married/steady partner | 73 | 20.6 | 1 | 1.4 | 72 | 98.6 | 0.32 |
| Single/divorced/widower | 282 | 79.4 | 13 | 4.6 | 269 | 95.4 | |
| Sleep on the streets | |||||||
| Yes | 204 | 57.5 | 6 | 2.9 | 198 | 97.1 | 0.26 |
| No | 151 | 42.5 | 8 | 5.3 | 143 | 94.7 | |
| Daily alcohol consumptionc | |||||||
| Yes | 108 | 31.1 | 8 | 7.4 | 100 | 92.6 | 0.03 |
| No | 239 | 68.9 | 6 | 2.5 | 233 | 97.5 | |
| Injection drug used | |||||||
| Yes | 31 | 8.9 | 2 | 6.5 | 29 | 93.5 | 0.47 |
| No | 317 | 91.1 | 12 | 3.8 | 305 | 96.2 | |
| Cocaine usea | |||||||
| Yes | 33 | 9.3 | 1 | 3.0 | 32 | 97.0 | 1.00 |
| No | 322 | 90.7 | 13 | 4.0 | 309 | 96.0 | |
| Marijuana smokinga | |||||||
| Yes | 104 | 29.3 | 4 | 3.8 | 100 | 96.2 | 1.00 |
| No | 251 | 70.7 | 10 | 4.0 | 241 | 96.0 | |
| Crack cocaine usea | |||||||
| Yes | 97 | 27.3 | 5 | 5.2 | 92 | 94.8 | 0.47 |
| No | 258 | 72.7 | 9 | 3.5 | 249 | 96.5 | |
| Age of first sexual experienced | |||||||
| Median (interquartile range) | 14 | (13, 16) | |||||
| ≤14 years | 182 | 52.3 | 8 | 4.4 | 174 | 95.6 | 0.71 |
| >14 years | 166 | 47.7 | 6 | 3.6 | 160 | 96.4 | |
| Previous sexual violencee | |||||||
| Yes | 79 | 22.5 | 5 | 6.3 | 74 | 93.7 | 0.23 |
| No | 272 | 77.5 | 9 | 3.3 | 263 | 96.7 | |
| Sexual partners in lifetimef | |||||||
| Median (interquartile range) | 20 | (9, 60) | |||||
| ≤20 | 173 | 50.3 | 6 | 3.5 | 167 | 96.5 | 0.57 |
| >20 | 171 | 48.2 | 8 | 4.7 | 163 | 95.3 | |
| Condom use with a steady partnerg | |||||||
| Ever | 21 | 14.6 | 0 | – | 21 | 100.0 | 0.47 |
| Sometimes/never | 123 | 85.4 | 3 | 2.4 | 120 | 97.6 | |
| Condom with a casual partnerh | |||||||
| Ever | 79 | 45.7 | 2 | 2.5 | 77 | 97.5 | 0.54 |
| Sometimes/never | 94 | 54.3 | 4 | 4.3 | 90 | 95.7 | |
| Exchange sex for money or drugsi | |||||||
| Yes | 44 | 12.6 | 4 | 9.1 | 40 | 90.9 | 0.08 |
| No | 306 | 87.4 | 10 | 3.3 | 296 | 96.7 | |
| Sex with a sex workere | |||||||
| Yes | 233 | 66.4 | 9 | 3.9 | 224 | 96.1 | 0.86 |
| No | 118 | 33.6 | 5 | 4.2 | 113 | 95.8 | |
| Sex with a drug userj | |||||||
| Yes | 201 | 59.3 | 10 | 5.0 | 191 | 95.0 | 0.41 |
| No | 138 | 40.7 | 4 | 2.9 | 134 | 97.1 | |
| Sex with PLWH infection | |||||||
| Yes | 26 | 7.3 | 5 | 19.2 | 21 | 80.8 | < 0.001 |
| No | 329 | 92.7 | 9 | 2.7 | 320 | 97.3 | |
| Same-sex sexual activityc | |||||||
| Yes | 72 | 20.7 | 9 | 12.5 | 63 | 87.5 | < 0.001 |
| No | 275 | 79.3 | 5 | 1.8 | 270 | 98.2 | |
| Anal sexi | |||||||
| Yes | 181 | 51.7 | 10 | 5.5 | 171 | 94.5 | 0.13 |
| No | 169 | 48.3 | 4 | 2.4 | 165 | 97.6 | |
| Sharing of objects for personal use | |||||||
| Yes | 247 | 69.6 | 9 | 3.6 | 238 | 96.4 | 0.66 |
| No | 108 | 30.4 | 5 | 4.6 | 103 | 95.4 | |
| Previously incarceratede | |||||||
| Yes | 181 | 51.4 | 6 | 3.3 | 175 | 96.7 | 0.51 |
| No | 171 | 48.6 | 8 | 4.7 | 163 | 95.3 | |
| Tattoos/piercings | |||||||
| Yes | 149 | 42.0 | 9 | 6.0 | 140 | 94.0 | 0.08 |
| No | 206 | 58.0 | 5 | 2.4 | 201 | 97.6 | |
| Previous HIV testingi | |||||||
| Yes | 194 | 55.4 | 11 | 5.7 | 183 | 94.3 | 0.10 |
| No | 156 | 44.6 | 3 | 1.9 | 153 | 98.1 | |
| Previous syphilis testingk | |||||||
| Yes | 111 | 32.4 | 6 | 5.4 | 105 | 94.6 | 0.28 |
| No | 232 | 67.6 | 7 | 3.0 | 225 | 97.0 | |
| History of genital lesions | |||||||
| Yes | 18 | 5.1 | 2 | 11.1 | 16 | 88.9 | 0.16 |
| No | 332 | 94.9 | 12 | 3.6 | 320 | 96.4 | |
OR: odds ratio; 95% CI: 95% confidence interval; PLWH: people living with HIV.
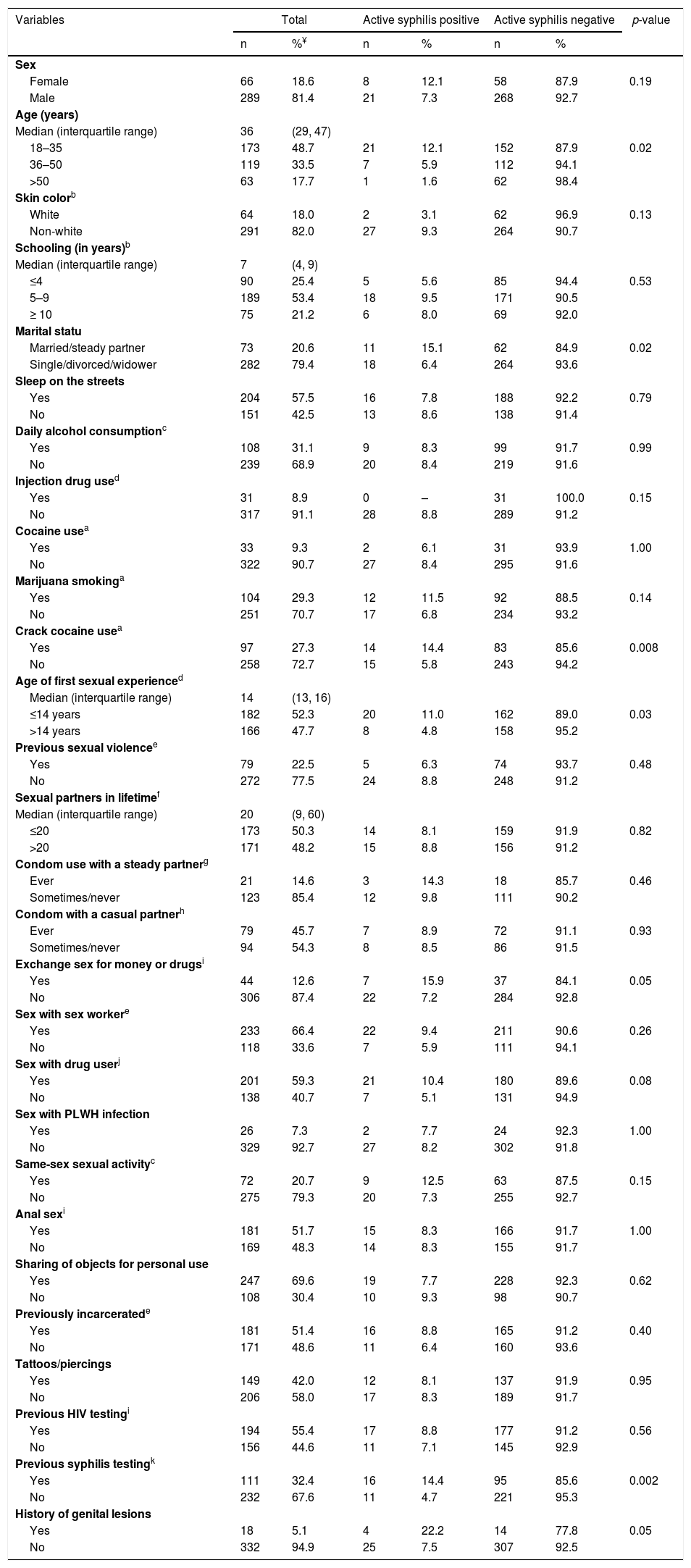
Univariate analysis of factors associated with the prevalence of active syphilis among 355 homeless people in Goiânia, Central-Western Brazil.
| Variables | Total | Active syphilis positive | Active syphilis negative | p-value | |||
|---|---|---|---|---|---|---|---|
| n | %¥ | n | % | n | % | ||
| Sex | |||||||
| Female | 66 | 18.6 | 8 | 12.1 | 58 | 87.9 | 0.19 |
| Male | 289 | 81.4 | 21 | 7.3 | 268 | 92.7 | |
| Age (years) | |||||||
| Median (interquartile range) | 36 | (29, 47) | |||||
| 18–35 | 173 | 48.7 | 21 | 12.1 | 152 | 87.9 | 0.02 |
| 36–50 | 119 | 33.5 | 7 | 5.9 | 112 | 94.1 | |
| >50 | 63 | 17.7 | 1 | 1.6 | 62 | 98.4 | |
| Skin colorb | |||||||
| White | 64 | 18.0 | 2 | 3.1 | 62 | 96.9 | 0.13 |
| Non-white | 291 | 82.0 | 27 | 9.3 | 264 | 90.7 | |
| Schooling (in years)b | |||||||
| Median (interquartile range) | 7 | (4, 9) | |||||
| ≤4 | 90 | 25.4 | 5 | 5.6 | 85 | 94.4 | 0.53 |
| 5–9 | 189 | 53.4 | 18 | 9.5 | 171 | 90.5 | |
| ≥ 10 | 75 | 21.2 | 6 | 8.0 | 69 | 92.0 | |
| Marital statu | |||||||
| Married/steady partner | 73 | 20.6 | 11 | 15.1 | 62 | 84.9 | 0.02 |
| Single/divorced/widower | 282 | 79.4 | 18 | 6.4 | 264 | 93.6 | |
| Sleep on the streets | |||||||
| Yes | 204 | 57.5 | 16 | 7.8 | 188 | 92.2 | 0.79 |
| No | 151 | 42.5 | 13 | 8.6 | 138 | 91.4 | |
| Daily alcohol consumptionc | |||||||
| Yes | 108 | 31.1 | 9 | 8.3 | 99 | 91.7 | 0.99 |
| No | 239 | 68.9 | 20 | 8.4 | 219 | 91.6 | |
| Injection drug used | |||||||
| Yes | 31 | 8.9 | 0 | – | 31 | 100.0 | 0.15 |
| No | 317 | 91.1 | 28 | 8.8 | 289 | 91.2 | |
| Cocaine usea | |||||||
| Yes | 33 | 9.3 | 2 | 6.1 | 31 | 93.9 | 1.00 |
| No | 322 | 90.7 | 27 | 8.4 | 295 | 91.6 | |
| Marijuana smokinga | |||||||
| Yes | 104 | 29.3 | 12 | 11.5 | 92 | 88.5 | 0.14 |
| No | 251 | 70.7 | 17 | 6.8 | 234 | 93.2 | |
| Crack cocaine usea | |||||||
| Yes | 97 | 27.3 | 14 | 14.4 | 83 | 85.6 | 0.008 |
| No | 258 | 72.7 | 15 | 5.8 | 243 | 94.2 | |
| Age of first sexual experienced | |||||||
| Median (interquartile range) | 14 | (13, 16) | |||||
| ≤14 years | 182 | 52.3 | 20 | 11.0 | 162 | 89.0 | 0.03 |
| >14 years | 166 | 47.7 | 8 | 4.8 | 158 | 95.2 | |
| Previous sexual violencee | |||||||
| Yes | 79 | 22.5 | 5 | 6.3 | 74 | 93.7 | 0.48 |
| No | 272 | 77.5 | 24 | 8.8 | 248 | 91.2 | |
| Sexual partners in lifetimef | |||||||
| Median (interquartile range) | 20 | (9, 60) | |||||
| ≤20 | 173 | 50.3 | 14 | 8.1 | 159 | 91.9 | 0.82 |
| >20 | 171 | 48.2 | 15 | 8.8 | 156 | 91.2 | |
| Condom use with a steady partnerg | |||||||
| Ever | 21 | 14.6 | 3 | 14.3 | 18 | 85.7 | 0.46 |
| Sometimes/never | 123 | 85.4 | 12 | 9.8 | 111 | 90.2 | |
| Condom with a casual partnerh | |||||||
| Ever | 79 | 45.7 | 7 | 8.9 | 72 | 91.1 | 0.93 |
| Sometimes/never | 94 | 54.3 | 8 | 8.5 | 86 | 91.5 | |
| Exchange sex for money or drugsi | |||||||
| Yes | 44 | 12.6 | 7 | 15.9 | 37 | 84.1 | 0.05 |
| No | 306 | 87.4 | 22 | 7.2 | 284 | 92.8 | |
| Sex with sex workere | |||||||
| Yes | 233 | 66.4 | 22 | 9.4 | 211 | 90.6 | 0.26 |
| No | 118 | 33.6 | 7 | 5.9 | 111 | 94.1 | |
| Sex with drug userj | |||||||
| Yes | 201 | 59.3 | 21 | 10.4 | 180 | 89.6 | 0.08 |
| No | 138 | 40.7 | 7 | 5.1 | 131 | 94.9 | |
| Sex with PLWH infection | |||||||
| Yes | 26 | 7.3 | 2 | 7.7 | 24 | 92.3 | 1.00 |
| No | 329 | 92.7 | 27 | 8.2 | 302 | 91.8 | |
| Same-sex sexual activityc | |||||||
| Yes | 72 | 20.7 | 9 | 12.5 | 63 | 87.5 | 0.15 |
| No | 275 | 79.3 | 20 | 7.3 | 255 | 92.7 | |
| Anal sexi | |||||||
| Yes | 181 | 51.7 | 15 | 8.3 | 166 | 91.7 | 1.00 |
| No | 169 | 48.3 | 14 | 8.3 | 155 | 91.7 | |
| Sharing of objects for personal use | |||||||
| Yes | 247 | 69.6 | 19 | 7.7 | 228 | 92.3 | 0.62 |
| No | 108 | 30.4 | 10 | 9.3 | 98 | 90.7 | |
| Previously incarceratede | |||||||
| Yes | 181 | 51.4 | 16 | 8.8 | 165 | 91.2 | 0.40 |
| No | 171 | 48.6 | 11 | 6.4 | 160 | 93.6 | |
| Tattoos/piercings | |||||||
| Yes | 149 | 42.0 | 12 | 8.1 | 137 | 91.9 | 0.95 |
| No | 206 | 58.0 | 17 | 8.3 | 189 | 91.7 | |
| Previous HIV testingi | |||||||
| Yes | 194 | 55.4 | 17 | 8.8 | 177 | 91.2 | 0.56 |
| No | 156 | 44.6 | 11 | 7.1 | 145 | 92.9 | |
| Previous syphilis testingk | |||||||
| Yes | 111 | 32.4 | 16 | 14.4 | 95 | 85.6 | 0.002 |
| No | 232 | 67.6 | 11 | 4.7 | 221 | 95.3 | |
| History of genital lesions | |||||||
| Yes | 18 | 5.1 | 4 | 22.2 | 14 | 77.8 | 0.05 |
| No | 332 | 94.9 | 25 | 7.5 | 307 | 92.5 | |
OR: odds ratio; 95% CI: 95% confidence interval; PLWH: people living with HIV.
The univariate analysis of potential factors associated with HIV infection and syphilis showed nine (age, daily alcohol consumption, exchange sex for money or drugs, sex with PLWH infection, same-sex sexual activity, anal sex, tattoos/piercings, previous HIV testing, history of genital lesions) and 14 (sex, age, skin color, marital status, injection drug use, marijuana smoking, crack cocaine use in the past 6 months, age of first sexual experience, exchange sex for money or drugs, sex with a drug user, same-sex sexual activity, previous syphilis testing, history of genital lesions) variables with p-values<0.20, respectively, and were included in multiple regression models.
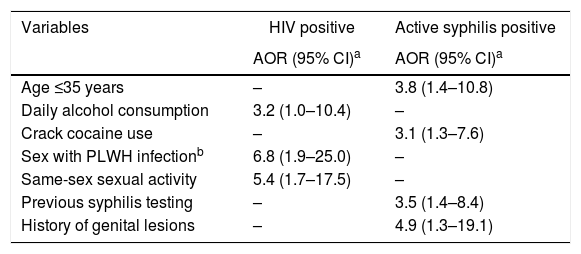
According to multiple logistic regression models, participants who reported daily alcohol consumption were 3.2-fold (95% CI: 1.0–10.4) more likely to be infected with HIV-1 compared to those did not report daily alcohol consumption. Those who reported having sex with an HIV-infected individual had a 6.8-fold (95% CI: 1.9–25.0) higher chance of being HIV-1 infected than those who did not. In addition, participants who reported sex with an individual of the same sex had a 5.4 times (95% CI: 1.7–17.5) higher chance of being infected with HIV-1 than those who did not report such sexual practice. Additionally, age ≤35 years (AOR: 3.8, 95% CI: 1.4–10.8), report of previous syphilis testing (AOR 3.5, 95% CI: 1.4–8.4), history of genital lesions (AOR 4.9, 95% CI: 1.3–19.1), and crack use in the past six months (AOR 3.1, 95% CI: 1.3–7.6) were predictors of active syphilis among the shelter-homeless investigated in this study (Table 3).
Multiple regression analysis of factors associated with human immunodeficiency virus infection and active syphilis among 355 homeless people in Goiânia, Central-Western Brazil.
| Variables | HIV positive | Active syphilis positive |
|---|---|---|
| AOR (95% CI)a | AOR (95% CI)a | |
| Age ≤35 years | – | 3.8 (1.4–10.8) |
| Daily alcohol consumption | 3.2 (1.0–10.4) | – |
| Crack cocaine use | – | 3.1 (1.3–7.6) |
| Sex with PLWH infectionb | 6.8 (1.9–25.0) | – |
| Same-sex sexual activity | 5.4 (1.7–17.5) | – |
| Previous syphilis testing | – | 3.5 (1.4–8.4) |
| History of genital lesions | – | 4.9 (1.3–19.1) |
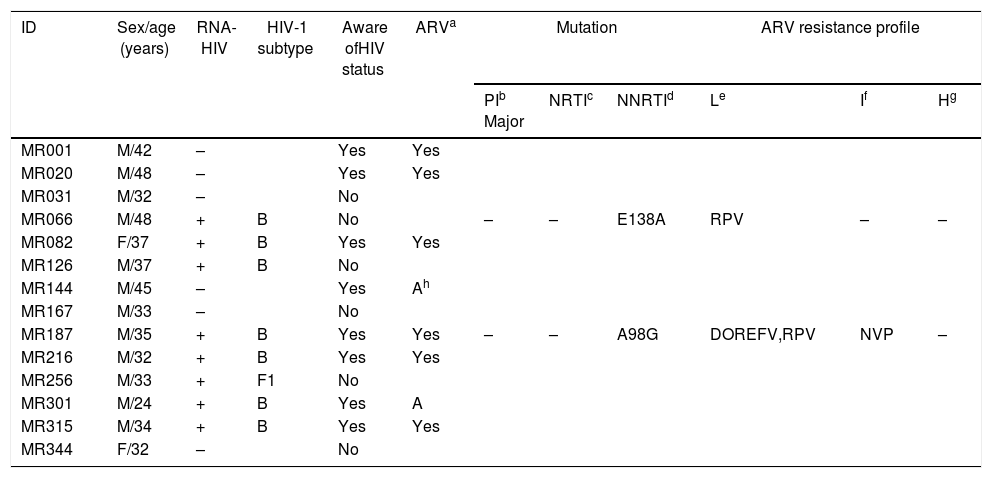
Fourteen individuals were anti-HIV-1 positive (3.9%; 95% CI: 2.3–6.4), of whom eight knew their serological status. Of these, six of the eight participants reported to be on ARV therapy (ART), and two of the eight participants had abandoned treatment. In eight out of the 14 anti-HIV-1 positive samples, the sequences of the PR and RT regions of the HIV-1 pol gene were amplified, and seven of the eight samples were sequenced successfully, with seven samples classified as subtype B and one as subtype F1. Two homeless men (ID# MR066 and MR187) were infected with HIV-1 isolates that presented single mutations associated with non-nucleoside reverse transcriptase inhibitors (NNRTIs) (E138A, A98G) (Table 4). Both were males aged 48 and 35 years. One of them (ID# MR066) was ARV-naive and showed a low ARV resistance profile to rilpivirine. The other participant (ID# MR187) reported previous ARV treatment and was infected with HIV-1 isolate with low resistance profile to efavirenz and rilpivirine and intermediate resistance to nevirapine. However, data on the ART regimen and duration of therapy were not available.
Demographic and molecular characteristics of 14 anti-human immunodeficiency virus-1-positive homeless individuals in Goiania, Central-Western Brazil.
| ID | Sex/age (years) | RNA-HIV | HIV-1 subtype | Aware ofHIV status | ARVa | Mutation | ARV resistance profile | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| PIb Major | NRTIc | NNRTId | Le | If | Hg | ||||||
| MR001 | M/42 | – | Yes | Yes | |||||||
| MR020 | M/48 | – | Yes | Yes | |||||||
| MR031 | M/32 | – | No | ||||||||
| MR066 | M/48 | + | B | No | – | – | E138A | RPV | – | – | |
| MR082 | F/37 | + | B | Yes | Yes | ||||||
| MR126 | M/37 | + | B | No | |||||||
| MR144 | M/45 | – | Yes | Ah | |||||||
| MR167 | M/33 | – | No | ||||||||
| MR187 | M/35 | + | B | Yes | Yes | – | – | A98G | DOREFV,RPV | NVP | – |
| MR216 | M/32 | + | B | Yes | Yes | ||||||
| MR256 | M/33 | + | F1 | No | |||||||
| MR301 | M/24 | + | B | Yes | A | ||||||
| MR315 | M/34 | + | B | Yes | Yes | ||||||
| MR344 | F/32 | – | No | ||||||||
M: male; F: female.
Of the total participants, 78 (22.0%) (95% CI: 17.9–26.5) were positive for syphilis in the rapid test, and 29 (8.2%; 95% CI: 5.6–11.4) were also positive for VDRL test, suggesting active syphilis. In addition, 14 of the 29 (48.3%) participants showed VDRL titer ≥1:8, indicating high potential for T. pallidum transmission. Five out of the 14 anti-HIV-1 positive homeless individuals were exposed to T. pallidum. Two of them were also VDRL positive.
DiscussionThe prevalence of HIV varies among homeless people worldwide, depending on the characteristics of the population studied and study design.2 In this study, the prevalence of HIV-1 infection among homeless people was 3.9% (95% CI: 2.3–6.4). This is 6.5-fold greater than the estimated prevalence for the general Brazilian population, highlighting that homeless people have a higher risk of acquiring HIV infection.21 Furthermore, this prevalence is within the observed variation in other Brazilian studies among homeless people: from 1.24% (95% CI: 0.57–2.69) to 4.9% (95% CI: 3.9–6.2).16–18
Investigations have reported that housing status plays a role in the effectiveness of HIV treatment.22,23 Unstable, inadequate housing or homelessness seem to increase HIV-related disparities. In general, adherence to ART is poor among homeless people, making them vulnerable to HIV-1 isolates resistant to ART.23 In fact, eight of the 14 HIV-infected individuals knew their diagnosis and reported having had started ART treatment. One participant (ID# MR187) was infected with an HIV-1 isolate with low and intermediate resistance profile to NNRTI. However, he was unable to provide information about his ART regimen and the duration of treatment. Furthermore, two homeless participants reported haing abandoned ART treatment; however, no mutation associated with drug resistance was detected.
Interestingly, two participants (ID# MR066 and MR187) showed singleton mutations conferring resistance to rilpivirine, a second-generation NNRTI not available in our country. It is noteworthy that MR066 was ARV-naive. Other Brazilian authors have also reported mutations with low resistance profile to this NNRTI among naive patients from Maranhão State, which is located in the northeast region of Brazil,24 the same state of origin of participant MR066, highlighting the need to monitor TDR levels.
Only HIV-1 subtypes B and F1 were detected among the homeless people in our study setting, with a predominance subtype B. These HIV-1 subtypes have been found in the Central-West region25,26 and other regions of Brazil.27
In this study, a lifetime syphilis prevalence of 22.0% (95% CI: 17.9–26.5) was estimated among the homeless. Investigations conducted in a Brazilian sentinel population showed that syphilis prevalence was lower than that found among homeless. Miranda et al.28 reported that the prevalence of lifetime syphilis among pregnant women was 0.4%, and Ribeiro et al.29 reported a 0.5% prevalence among male conscripts, supporting the idea that homeless individuals are at higher risk for contracting STIs. In addition, a high prevalence of VDRL positivity was found (8.2%) (95% CI: 5.6–11.4), and half of the individuals showed high titers (VDRL ≥1:8), evidencing the high burden of this infection and the potential for syphilis dissemination among homeless and vulnerable individuals.
Although HIV-1 infection and syphilis share common transmission modes, the present study revealed different predictors for these infections. A stronger association was found between daily alcohol consumption and HIV-1 infection. Investigations have reported the negative effects of alcohol consumption on HIV infection, which include risky sexual behavior (poor adaptation to or negotiation of condoms during sexual intercourse) and poor ART adherence.30 Additionally, long-term alcohol use has been associated with more advanced HIV disease.31
Sex with PLWH infection and same-sex sexual intercourse were predictors of HIV-1 infection, indicating that sexual transmission is a major HIV-1 transmission mode among the homeless people studied. This finding likely reflects a significant lower frequency of condom use during sexual intercourse. In fact, without the use of condoms during sexual intercourse, it is estimated that the risk per act of acquiring HIV infection from an infected individual ranges from 0.04% during insertive penile–vaginal intercourse to 1.38% during receptive anal intercourse.32 Nowadays, the HIV pre-exposure prophylaxis for HIV prevention could benefit this at-risk group.33
Crack cocaine use was strictly associated with syphilis. Other studies have also shown high frequencies of syphilis among crack users, and a synergism between crack and syphilis has been suggested.34,35 In fact, investigations from the 1980s showed that the introduction of cracks in some regions of the world was followed by an increase in syphilis cases, indicating a temporal relationship between them.36–38 Senã et al.39 reported a syphilis outbreak in a rural region of North Carolina, USA, from January 2001 to February 2002, during which the use of crack and/or sex in exchange for crack cocaine was the major factor responsible for the outbreak. In Brazil, cracks surged in early 1990s on the streets of São Paulo City, which is located in the southeast region of Brazil,40 and spread throughout the country.41 Some Brazilian studies conducted in crack users have also shown high frequencies of syphilis in this population.42,43 Therefore, further investigations are necessary to identify the real role of crack cocaine in the transmission of syphilis.
Our study found a strict association between young adult age and active syphilis, which is concurrent with the existing literature. In general, these individuals are more sexually active and therefore have more opportunities for exposure to sexually transmitted microorganisms.44
Individuals who reported previous syphilis testing and those with a history of genital lesions had a 2.6- and 4.9-fold greater chance of having active syphilis, respectively. These findings were not surprising since this population repeatedly engages in high-risk sexual activity, and individuals with symptomatic primary syphilis usually develop genital lesions.45
Some limitations must be considered when interpreting our results. First, the cross-sectional design in which the outcome and exposure variables are collected simultaneously makes it impossible to establish causality of the factors studied. The study was conducted among individuals who are “shelter-homeless” (in a public shelter); therefore, the findings may not be representative of the totality of homeless people in Goiânia City. However, the characteristics of the individuals examined were similar to those presented in a national homeless study,14 suggesting external validity. Although interviewers ensured anonymity of responses, and interviews were conducted in private settings, several questions in the questionnaire dealt with issues involving intimacy. Those responses may not match to what is expected by societal moral standards; therefore, participants may have felt uncomfortable responding to them freely.
In view of the increasing number of people on the streets, consequently at higher risk of exposure to HIV infection and other STIs,4 it is imperative to consider the principle of integrality, equity, and universality13 as a way of reorienting professional practices, healthcare services, and public policies that can have an impact on the health and quality of life of these individuals. In this sense, it is necessary to understand the complexity and multi-causality that involve the process of living on the streets, without dissociating it from its historical, social, and political context.46 Therefore, it is necessary to organize healthcare services in integrated networks, articulating emergency units with primary care, to guarantee continuity of care.13 No less important, healthcare services must work in intra- and inter-sectoral networks, articulating social action, education, justice, culture, and human rights, aiming not only at promoting health but also at reinserting that individual in society.46
The present findings highlight the need for effective policies to prevent and control STIs among homeless people, which should include testing, counseling, and treatment for STIs. The low adherence to ART and detection of two homeless participants infected with HIV-1 isolate mutations potentially associated with drug resistance to ARV not available in our country should be paid careful attention.
FundingThis study was funded by the United Nations Office on Drugs and Crime, in partnership with the Ministry of Health-STD/HIV/AIDS Coordination and Viral Hepatitis-call: 003/2013.
Declaration of interest statementThe authors declare no conflicts of interest.
The authors thank all homeless individuals who participated in this study.