The pandemic of COVID-19 brought to the world an unprecedented challenge. This single center observational study aimed to evaluate the impact of staff preparedness by comparing the outcomes between two intensive care units (ICUs) from a hospital that had to expand ICU beds to deal with an incremented volume of critical patients. Patients consecutively admitted to these ICUs with suspected COVID-19, from March 1st until April 30th, 2020, were included. Both ICUs attended a similar population and had the same facilities, what differed was the staff: one previously well-established (ICU-1) and another recently assembled (ICU-2). 114 patients with severe respiratory syndrome were included. In-hospital mortality was 40%. Compared with patients in the well-established ICU-1, patients in the recently assembled ICU-2 were older (54 versus 61.5, p=0.045), received more antibiotics (93% versus 98%, p=0.001) and chloroquine/hydroxychloroquine 6% versus 30%, p=0.001), had a higher proportion of invasive mechanical ventilation (44% versus 52%, p=0.008) and had greater in-hospital mortality (30% versus 50%, p=0.017). The proportion of patients considered at high risk for death according to PSI was similar between the two ICU populations. Age ≥ 60 years (adjusted OR 2.33; 95% CI 1.02-5.31), need of invasive mechanical ventilation (adjusted OR 2.79; 95% CI 1.22-6.37), and ICU type (recently assembled) (adjusted OR 2.38; 95% CI 1.04-5.44) were independently associated with in-hospital mortality . This finding highlights the importance of developing support strategies to improve preparedness of staff recently assembled to deal with emergencies.
As of December 2019, the first cases of COVID-19, a disease caused by the new coronavirus SARS-CoV-2, have been reported to the World Health Organization (WHO).1 In March 2020, WHO declared a pandemic2 and since then the world is facing an unprecedented challenge. Several regions have been forced to increase intensive care unit (ICU) capacity in a short period of time to deal with an expressive number of critically ill patients.3,4
Latin America followed this trend, and the populous city of São Paulo began to represent one epicenter of the disease in the world, with 90,293 suspected COVID-19 cases (18,438 confirmed)5 and 3622 deaths (1665 confirmed)6 reported by the end of April, 2020.
During a hospital surge, huge increases of ICU capacity may lead to degraded capability and possible modification in the standard of care.7 Studies investigating different strategies to deal with ICU capacity increase are scarce, but important to guide policy makers.
This study aimed to evaluate the impact of staff preparedness by comparing patients outcomes between two ICUs from the same hospital, with the same facilities and similar attended population, one previously well-established and another recently assembled to deal with a markedly increased volume of patients brought by COVID-19 pandemic.
Material and methodsStudy design and participantsThis was an observational cohort study with retrospective collection of data, conducted at Instituto de Infectologia Emílio Ribas (IIER), São Paulo-SP, Brazil.
This center is a referral hospital to infectious diseases with a large experience in the management of critically ill patients with endemic and epidemic infectious diseases such as AIDS, yellow fever, meningococcal disease, leptospirosis, Brazilian spotted fever, and influenza (H1N1) pdm09.
During the study period, IIER was designated for triage and treatment of severe COVID-19 cases. Patients could spontaneously seek medical attention at the Emergency Room (ER) or be transferred from other hospitals in the State of São Paulo.
All patients admitted to these ICUs with suspected COVID-19, from March 1st through April 30th, 2020 were included. Suspected COVID-19 patients were those meeting criteria for severe acute respiratory syndrome: fever (referred or measured) AND cough or sore throat AND dyspnea or oxygen saturation < 95% or respiratory distress.8,9
The ER treatment was prescribed at the attending physician's discretion, as well as the evaluation of the need of intensive care. While in the ICUs, diagnostic and therapeutic procedures were the responsibility of each ICU team.
The experimental agent chloroquine/hydroxychloroquine could be prescribed as compassionate treatment for severe and critical cases, with consent from patient or family, following Brazilian Ministry of Health orientation at the time.
The first institutional clinical management guideline for COVID-19 patients was released on April 12, 2020. This guideline advised that patients with oxygen saturation < 90% or respiratory rate > 30 incursions per minute or hypotension or altered level of consciousness were to be admitted to an ICU. There was a recommendation for introducing antibacterial agents and neuraminidase inhibitors for all patients but there was no mention about steroids.
To deal with the increased number of critical patients, the number of ICU beds at IIER more than doubled, going from 12 to 30 beds. The allocation of patients between the two ICUs was by chance, depending on availability of beds. An institutional bed management team controlled the vacant beds and requests for ICU admission.
The ICUs were in the same building and had similar standards: same structure, same access to hospital facilities (including laboratory, radiology, and nutrition services) and same equipment. The staff/patient ratio was also similar between the two ICUs.
The only discrepancy was the staff, consisting of physicians, nurses, speech therapists and physiotherapists: 10 beds remained under the responsibility of government-employed staff with large experience in infectious diseases (ICU-1) and 20 beds were undertaken by a recently assembled outsourced team (ICU-2). The majority of the physicians in ICU-1 team were infectious diseases specialists and only ICU-1 had an educational program for infectious diseases residents.
This is a sub-analysis of a largest study proposed by World Health Organization for global COVID-19 clinical characterization10 that is been conducted at the Institute by our team.
The National Research and Ethics Commission (CONEP) approved this study (CAAE 30632820.2.0000.0061). The requirement for informed consent was waived because of the observational study design. Our protocol was performed in accordance with the relevant guidelines and regulations.
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Data collectionData was collected through a standardized case record form (CRF) developed by the International Severe Acute Respiratory and Emerging Infection Consortium (ISARIC) and WHO in Research Electronic Data Capture software (REDCap, Vanderbilt University).
We obtained demographic data, comorbidities, vital signs, radiologic findings, laboratory results and instituted therapy within 24 hours of admission. Results of viral respiratory pathogens tested were recorded.
Severity was evaluated using the Pneumonia Severity Index (PSI).11 Data was collected retrospectively through review of hospital charts and the epidemiology service database.
The assessed outcome was in-hospital mortality. Follow up time was censored on June 13, 2020.
Statistical analysesContinuous variables were reported as medians and interquartile range (IQR). Categorical variables were summarized as counts and percentages. Missing data were not imputed.
Differences between the two ICUs were assessed using the chi-squared or Fisher's exact or Mann-Whitney test, as appropriate.
Outcome was assessed with unadjusted and multivariate models, using logistic regression. Our model included variables that presented p<0.20 on unadjusted analysis and had no significant correlation with other variables. The model was generated by backward stepwise regression. The fitness of our model was evaluated by the Hosmer-Lemeshow test. Results are presented as odds ratios (OR).
The level of significance was set at 0.05 (two-tailed). Analyses were performed with Statistical Package for Social Science (version 25; SPSS, Chicago, IL).
ResultsIn this study, 114 cases with severe acute respiratory syndrome admitted to one of the two ICUs were included, 54 (47%) admitted to the well-established ICU-1 and 60 (53%) to the recently assembled ICU-2.
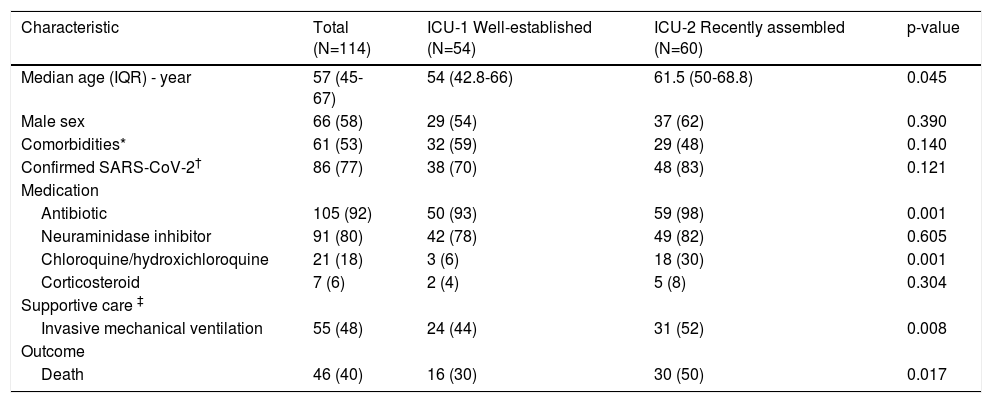
The clinical characteristics of those patients are summarized in Table 1. The median age was 57 years, with a male predominance. More than half of patients had at least one comorbidity (hypertension, diabetes, or obesity).
Characteristics of 114 patients with severe acute respiratory syndrome admitted to intensive care unit.
| Characteristic | Total (N=114) | ICU-1 Well-established (N=54) | ICU-2 Recently assembled (N=60) | p-value |
|---|---|---|---|---|
| Median age (IQR) - year | 57 (45-67) | 54 (42.8-66) | 61.5 (50-68.8) | 0.045 |
| Male sex | 66 (58) | 29 (54) | 37 (62) | 0.390 |
| Comorbidities* | 61 (53) | 32 (59) | 29 (48) | 0.140 |
| Confirmed SARS-CoV-2† | 86 (77) | 38 (70) | 48 (83) | 0.121 |
| Medication | ||||
| Antibiotic | 105 (92) | 50 (93) | 59 (98) | 0.001 |
| Neuraminidase inhibitor | 91 (80) | 42 (78) | 49 (82) | 0.605 |
| Chloroquine/hydroxichloroquine | 21 (18) | 3 (6) | 18 (30) | 0.001 |
| Corticosteroid | 7 (6) | 2 (4) | 5 (8) | 0.304 |
| Supportive care ‡ | ||||
| Invasive mechanical ventilation | 55 (48) | 24 (44) | 31 (52) | 0.008 |
| Outcome | ||||
| Death | 46 (40) | 16 (30) | 30 (50) | 0.017 |
Data are n (%) unless otherwise specified. IQR: interquartile range
Compared with the well-established ICU-1, patients in the recently assembled ICU-2 were older (median age of 61.5 versus 54 in well-established ICU-1, p=0.045).
According to PSI, 47 (41%) patients were considered at high risk for death and other adverse outcomes, without significant difference between the two ICU populations (23 patients, 43% in the well-established ICU-1 versus 24 patients, 40% in the recently assembled ICU-2, p=0.779).
Data on respiratory pathogens were available for 112 patients, of whom 86 (77%) had SARS-CoV-2 confirmed by polymerase chain reaction (PCR) and 4 (4%) had influenza detected by PCR. There was no confirmed mixed infection by these two viruses. One patient had confirmed influenza B and a Streptococcus pneumoniae bacteraemia. 21 (19%) patients had no pathogen identification.
Within 24 hours of admission, most patients received an antibacterial agent (105, 92%) and a neuraminidase inhibitor (91, 80%). Experimental agents chloroquine or hydroxychloroquine were administered to 19 (18%) patients. Corticosteroids were prescribed to 7 (6%) patients.
There were significant differences on therapy institution between the two ICUs: antibiotics were administered more frequently in the recently assembled ICU-2 (59 patients, 98% versus 50 patients, 93%, p=0.001), as well as chloroquine/hydroxychloroquine prescription (3 patients, 6% in ICU-1 versus 18 patients, 30% in ICU-2, p=0.001).
Supportive care with invasive mechanical ventilation was needed for 55 (48%) patients, with a higher proportion in the recently assembled ICU-2 (24 patients, 44% in ICU-1 versus 31 patients, 52% in ICU-2, p=0.008).
All patients had at least 30 days of follow-up. As of June 13, 2020, 63 (55%) patients had been discharged from hospital, 3 (3%) remained in hospital and 46 (40%) patients had died. The median length of hospitalization was 11 days (IQR 6 – 21), excluding the three patients remaining in hospital by the end of follow-up.
In-hospital mortality rate was 40%, greater in the recently assembled ICU-2 than in the well-established ICU-1 (30% in ICU-1 versus 50% in ICU-2, p=0.017).
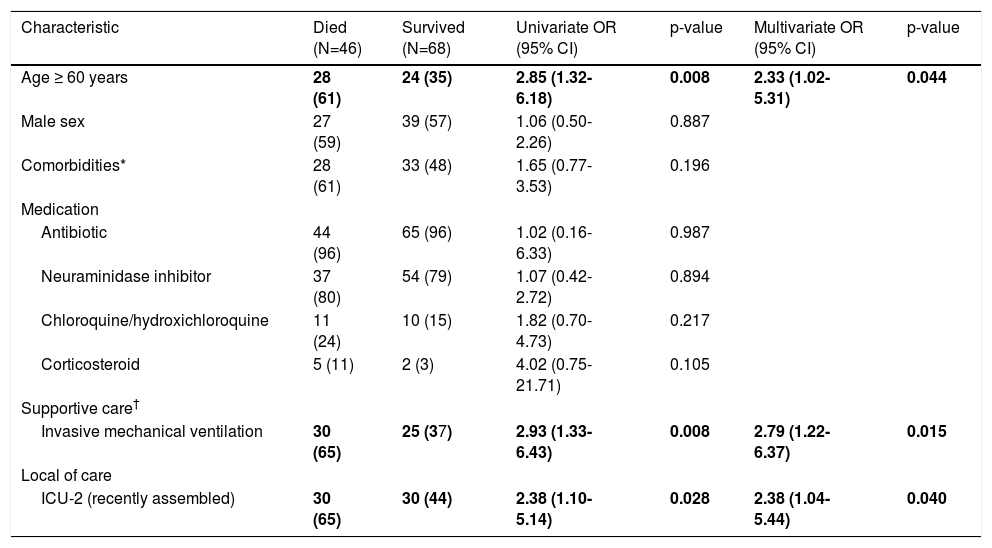
Age ≥ 60 years, mechanical ventilation, and ICU type (recently assembled) were risk factors for in-hospital mortality (Table 2). Since elderly is a well described risk factor for mortality by COVID-19,12,13 we categorized age (cut-off: 60 years).
Risk factors for in-hospital mortality of 114 patients with severe acute respiratory syndrome admitted to intensive care unit.
| Characteristic | Died (N=46) | Survived (N=68) | Univariate OR (95% CI) | p-value | Multivariate OR (95% CI) | p-value |
|---|---|---|---|---|---|---|
| Age ≥ 60 years | 28 (61) | 24 (35) | 2.85 (1.32-6.18) | 0.008 | 2.33 (1.02-5.31) | 0.044 |
| Male sex | 27 (59) | 39 (57) | 1.06 (0.50-2.26) | 0.887 | ||
| Comorbidities* | 28 (61) | 33 (48) | 1.65 (0.77-3.53) | 0.196 | ||
| Medication | ||||||
| Antibiotic | 44 (96) | 65 (96) | 1.02 (0.16-6.33) | 0.987 | ||
| Neuraminidase inhibitor | 37 (80) | 54 (79) | 1.07 (0.42-2.72) | 0.894 | ||
| Chloroquine/hydroxichloroquine | 11 (24) | 10 (15) | 1.82 (0.70-4.73) | 0.217 | ||
| Corticosteroid | 5 (11) | 2 (3) | 4.02 (0.75-21.71) | 0.105 | ||
| Supportive care† | ||||||
| Invasive mechanical ventilation | 30 (65) | 25 (37) | 2.93 (1.33-6.43) | 0.008 | 2.79 (1.22-6.37) | 0.015 |
| Local of care | ||||||
| ICU-2 (recently assembled) | 30 (65) | 30 (44) | 2.38 (1.10-5.14) | 0.028 | 2.38 (1.04-5.44) | 0.040 |
Data are n (%) unless otherwise specified. Hosmer-Lemeshow test = 0.997
Comorbidities is a variable that presented p < 0.20 on unadjusted analysis and was tested in the multivariate model, but it was not independently associated with the outcome (p = 0.55) and acted as a confounding factors with age. Therefore, this variable was excluded from the final model.
As age is a component of the PSI calculation, we believe that if we had considered PSI and age in the same multivariate model, the PSI would act as a confounding factor, distorting the association between exposure (age) and outcome.
We decided to not include chloroquine or hydroxychloroquine and corticosteroid in the multivariate model, since they were prescribed for just a few patients.
In multivariate analysis, age ≥ 60 years (adjusted OR 2.33; 95% CI 1.02-5.31), mechanical ventilation need (adjusted OR 2.79; 95% CI 1.22-6.37), and ICU type (recently assembled) (adjusted OR 2.38; 95% CI 1.04-5.44) remained independently associated with in-hospital mortality.
DiscussionIn this study, age ≥ 60 years, need of invasive mechanical ventilation and ICU type (recently assembled) were independently associated with in-hospital mortality among patients with severe acute respiratory syndrome during the COVID-19 pandemic in São Paulo, Brazil.
São Paulo, Brazil's largest city, experienced a rapid and unprecedented growth in the need for ICU beds, leading to increased hospital capacity in a short period of time. ICUs of public hospitals of São Paulo reached 90% capacity, with risk of collapse of the health system.
In this study, the ICUs mortality rate was 40%. Coincidentally, a recent systematic review and meta-analysis of 24 observational studies carried-out in Asia, Europe and North America including 10,150 patients with COVID-19 who were admitted to ICUs reported a mortality rate of 41.6%, with no significant difference across geographic locations.14
There is scarce information about outcomes of patients admitted to ICUs in Latin America during COVID-19 pandemic. The Brazilian Intensive Medicine Association created a national registry to characterize the epidemiologic profile of the Brazilian ICUs. In this project, among 33,161 patients with COVID-19 admitted to Brazilian ICUs, between March 1 and July 22, 2020, the in-hospital mortality rate was 34.4% which was remarkably different in private (27.8%) and public (50.4%) hospitals.15 These differences were due in part to the fact that in public hospitals patients usually arrive in worse clinical condition and the threshold for admission to an ICU are usually stricter. Results of the present study suggest that better outcomes are possible in public hospitals.
The variables age ≥ 60 years and need of invasive mechanical ventilation were identified in our study as independent factors associated with mortality. A systematic review of 14 observational studies including 4,659 hospitalized patients with COVID-19 identified older age and baseline cardiometabolic diseases (particularly hypertension, diabetes and chronic heart disease/cardiovascular disease) as risk factors associated with mortality.12,13 As with other diseases, patients with COVID-19 admitted to ICUs are more likely to undergo invasive mechanical ventilation and be intubated and are more likely to die.
Interestingly, even though patients in the recently assembled ICU-2 were older and had an increased need for mechanical ventilation when compared with patients in the well-established ICU-1, ICU type was independently associated with in-hospital mortality, more than doubling the risk for those individuals admitted in the recently assembled ICU.
As there is no validated severity score for patients with COVID-19, we have chosen PSI for its broadened evaluation. Although patients in ICU-2 were older, had a major male predominance and had more comorbidities, the rate of patients with high risk for death and other adverse outcomes according to PSI did not show significant differences between ICUs, indicating that probably patients in ICU-1 had worst clinical condition, laboratory and radiologic findings than patients in ICU-2. As the patient allocation to each ICU was random, according to the availability of ICU beds, we expected the population to be similar, as well as the treatment received in the ER.
Our study has the differential of being able to explore the impact of staff preparedness on clinical outcome, since in our institution the strategy to deal with the COVID-19 emergency was to hire an external ICU medical team to take care of patients in the ICU beds that had been expanded.
Although we could expect that an experienced staff ought to have better treatment skills, our results shed light on the importance to take this variable into account when studying the possible determinants of death during an emergency.
For hospitals to effectively respond to the anticipated surge in critical care patients, the balance between space (ICU beds), staff (nurses, physicians, and respiratory therapists), and stuff (i.e., ventilators) needs to be maximized.16
Significant increases in the normal capacity of an ICU may occur, but at the cost of reducing performance and a possible modification in the standard of care.7
There are reports of increasing ICU capacity by utilizing alternate hospital sites and non-ICU staff under the supervision of trained critical care personnel.7
Our findings can inform policy makers that the capacity of trainning and supervising recently assembled teams must be strengthened, possibly by establishing mentorship and evaluation programs, besides developing detailed and clear treatment protocols.
In our institution, a clinical management protocol was released with weeks of delay from the first COVID-19 case, what could have contributed to discrepancies between medication usage between the ICUs.
Although this study was carried out before evidence showing benefit of using corticosteroids in COVID-19 cases who require supplemental oxygen, our CRF had a section for collecting information on the use of medications and already included a specific field for corticosteroids.
This study has some limitations inherent to observational monocentric design. However, we included consecutives cases, patients were rigorously selected, and data collection used an ISARIC tool. Despite having been carried-out in a single center, this study conducted a strict comparative assessment of the ICU type under similar hospital conditions.
Since no randomization tool was applied, there was a potential for selection bias which could have lead to the baseline characteristics differences between patients assigned to the two ICUs. The multivariate analysis adjusted for the most significant differences, but certainly randomized and multicenter studies could bring more strength to our findings.
Regardless of the absence of more detailed analysis of severity between the groups, we consider that the results shown may be useful, especially for scenarios like ours. Finally, in this study we evaluated an initial period of the pandemic and it is possible that better outcomes would be obtained over time,14 particularly in the recently assembled ICU, with standardization of clinical protocol, in addition to continuous education of the professionals.
In conclusion, we found that age ≥ 60 years, need of invasive mechanical ventilation, and ICU type (recently assembled) were independently associated with in-hospital mortality among patients with severe acute respiratory syndrome during the COVID-19 pandemic. This finding highlights the importance of developing support strategies for improve preparedness of staff recently assembled to deal with emergencies.
Authors' contributionsDr. Figueiredo-Mello, Dr. Sztajnbok, Dr. Freitas Ribeiro, Dr. Malaque, and Dr. Vidal has contributed to the conception and conduction of the work.
Dr. Freitas Ribeiro has conducted the data analysis and interpretation.
Dr. Figueiredo-Mello, Dr. Sztajnbok, Dr. Freitas Ribeiro, Dr. Malaque, and Dr. Vidal has written this manuscript.
Dr. Figueiredo-Mello, Dr. Cavalin, Dr. Lanza, Dr. Castanheira, Dr. Rego, Dr. Custodio, and Dr. Siqueira has contributed with data collection
Dr. Figueiredo-Mello has been the main advisor of the work, from conception of the study to revision of this manuscript.
We are grateful to Laura Merson, Matthew Hall, and Daniel Plotkin on behalf of ISARIC for the support and collaboration.