Hepatitis E virus (HEV) affects 20 million people worldwide, with 3.3 million cases and 56,000 deaths. The transmission is mainly by the fecal-oral route. Several studies have reported increased alanine aminotransferase (ALT) levels in association with viral hepatitis. This study evaluated the diagnosis of HEV infection among patients attending the emergency room (ER) of Hospital Beneficência Portuguesa (HBP) and Hospital São Paulo (HSP) in São Paulo, Brazil increased ALT levels (≥ 200 IU/L). From October 2018 to July 2019, 400 sera samples were collected from patients treated at the ER of HBP (n=200) and HSP (n=200). All samples were screened for HEV by RT-qPCR. 200 samples from HSP were tested for IgM of anti-Hepatitis A (HAV) and B (HBV) viruses, and total antibodies of Hepatitis C virus (HCV). Ninety samples (45 from each hospital), were tested for anti-HEV IgM antibodies. Patients aged under 1 to 91 years (mean = 46.29 ± 24.17, median = 48). ALT levels varied from 200 to 8,974 IU/l. 16 patients (4%) turned out positive for HEV by RT-qPCR (ALT levels = 299 to 698 IU/L). Of the 200 HSP patients, 18 (9%) were anti-HAV IgM reactive, 9 (4.5%) for anti-HBV IgM, and 7 (3.5%) for anti-HCV antibodies (ALT levels = 833 to 1918 IU/L). Two of 90 BPH patients (2.22%) were anti-HEV IgM reactive (ALT levels = 1502 to 3831 IU/L). This is the first Brazilian study evaluating patients with suspected HEV infection with increased ALT levels, which were higher than 12 and 60 times the normal upper limit, in the acute phase or for patients reactive for antibody detection, respectively. Liver damage could be minimized by implementing molecular diagnostic tests in the hospital routine.
Hepatitis E virus (HEV) is responsible for more than 50% of cases of self-limited acute hepatitis worldwide and is a public health problem.1-4 According to the World Health Organization (WHO), in 2015, approximately 20 million people were infected with HEV. Genotypes 1 and 4 of the HEV are of public health concern, as they have caused acute liver injury in approximately 3.3 million people and are responsible for about 56,600 deaths. This infection can also evolve into fulminant hepatitis. The reported data have shown a rate of around 20% of deaths among HEV infected women in the third trimester of pregnancy.5
HEV infection is more frequent in the age group between 15 to 40 years and affects more men than women. It is transmitted mainly the fecal-oral route through ingestion of contaminated water, raw or undercooked meat. It is considered an anthropozoonosis since some animals, such as domestic pig, wild boar, camel, deer, mollusks, and other species, are potential reservoirs.3,4,6,7 In addition, it can also be transmitted vertically by transfusion of blood and blood products, and among transplanted patients, eventually by contaminated grafts.8
Recent studies suggested that clinical manifestations are associated with biochemical evidence of infection, such as increased rates of transaminases, which are indicative factors for identifying and diagnosing viral hepatitis since these enzymes are known biomarkers of liver injury. It is possible to measure the human enzymes released through hepatic cell disruption, which provides relevant information regarding the extent of liver injury, severity, and course (acute or chronic).9
Several studies have reported cases of unexplained increase of alanine aminotransferase (ALT) in association with the classic clinical manifestations of viral hepatitis, and the most common hepatitis being ruled out through laboratory tests, suggesting the possibility of HEV infection in these cases.10-15
In this study, we have evaluated the diagnosis of HEV infection among patients with increased ALT levels who presented for care at the emergency departments of two hospitals in the city of São Paulo (Hospital Beneficência Portuguesa and Hospital São Paulo).
Materials and methodsThis cross-sectional study evaluated sera samples of patients with ALT levels greater than ≥ 200 IU/L who had looked for care at the emergency rooms of Hospital São Paulo - HSP (n=200) and Hospital Beneficência Portuguesa - HBP (n=200), from October 2018 to July 2019.
The cutoff point of Alanine aminotransferase (ALT)We hypothesized that liver function tests could serve as a screening test for HEV infection as already proposed.16 However, other studies have shown that using an ALT cutoff level above 100 IU/L would be a suitable strategy and, therefore, excluding other possible diagnostic results, it would be feasible to investigate the occurrence of HEV infection in these patients.9-12 Nonetheless, in order to reduce the crossing with other medical conditions, an ALT ≥ 200 IU/L was adopted.
Serological and molecular detectionSerological tests for detection of anti-HEV IgM antibodies were performed in 90 samples, 45 from each hospital, using the WANTAI HEV-IgM ELISA (Beijing Wantai Biological Pharmacy Enterprise, Beijing, China), according to the manufacturer's recommendations. In 200 serum samples from HSP patients, the presence of IgM of anti-Hepatitis A virus (HAV), total antibodies of Hepatites B virus (HBV) and antigen (anti‑HBsAg), and total antibodies of Hepatitis C virus (HCV), were investigated by using the Elecsys kits for COBAS E 411 Analyzer, respectively, as follow, according to the manufacturer's recommendations: Anti-HAV IgM, HBsAg II, Anti-HBc II and Anti-HCV II (Roche Diagnóstics Brazil, LTDA).
For molecular screening, RNA was purified from the serum of all 400 patients using the QIAamp Viral RNA Minikit (QIAGEN, Hilden, Germany) as recommended by the manufacturer. The molecular detection was performed only for HEV by one-step real-time reverse transcription-polymerase chain reaction (RT-qPCR) with the oligonucleotides described elsewhere for open reading frames (ORFs) 2 17 and 3.18 The RT-qPCR reaction consisted of a triplex single tube detection of HEV and human ribonuclease P (RNase P) as an internal control.19 A total of 25 uL of reaction volume was set up with AgPath-ID One-Step RT-PCR Reagents (Applied Biosystems, Austin, USA), containing 600 nM of each primer and 200 nM of probes for HEV ORFs 2 and 3, and 496 nM of each primer and 160 nM of the probe for RNase P target. Amplification of ORFs 2 and 3, and RNase P, were detected by FAM, VIC, and Cy5 fluorophores, respectively. The reactions were performed in a 7500 Real-Time PCR System (Applied Biosystems). Thermocycling conditions were 50°C for 10 min., followed by 45 cycles at 95°C for 15 sec and 58°C for 30 sec.
StatisticsCategorical variables were analyzed using the Chi-square test, Fisher's exact test, and continuous variables by Pearson's correlation test.
ResultsCharacteristics of the study populationThe 400 investigated patients had a mean age of 46.29 ± 24.17 years (median = 48 years), ranging from less than 1 to 91 years, 51.25% were men. The ALT levels varied from 200 to 8,974 IU/L.
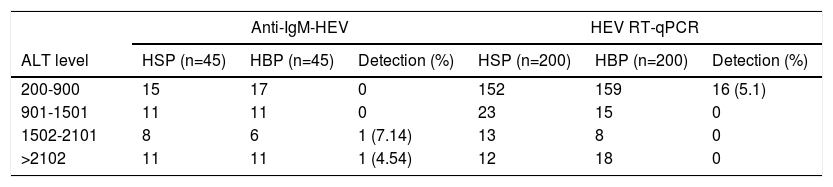
Molecular detection of HEVA total of 16 patients (4%) were positive for HEV by RT-qPCR. They had a mean age of 39.53 ± 24.10 years (median = 31), of whom 56.2% (9/16) were women, ALT levels ranged from 299 to 698 IU/l (mean = 441.87), not statistically different from the ALT mean level of non-HEV patients.
Serological tests for hepatitis EOut of the 90 patients from BPH who had anti-HEV IgM antibodies performed, two (2.22%) were reactive, one male and one female, aged 77 and 39 years-old, with ALT levels of 1505 and 3831 IU/l, respectively (Table 1).
Alanine aminotransferase levels related to hepatitis E virus antibody or genomic detection.
ALT, Alaninie aminotransferase. HSP, Hospital São Paulo. HBP, Hospital Beneficiência Portuguesa.
HEV, Hepatitis E vírus. RT-qPCR, real-time reverse transcriptase-polymerase chain reaction.
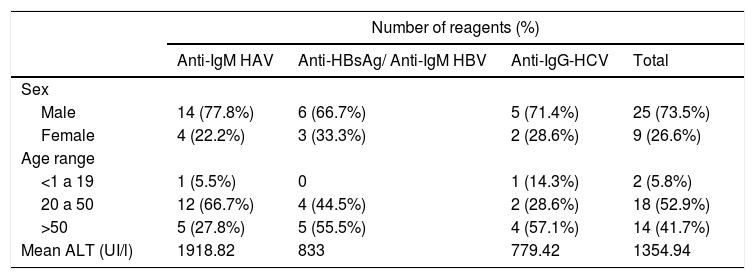
Of the 200 patients from HSP who were serologically tested for other hepatitis, 18 (9%) were reactive for anti-HAV IgM, 9 (4.5%) for anti‑HBsAg and anti-HBV IgM/IgG, and 7 (3.5%) for anti-HCV antibodies. Two of them were reactive for two viruses: a 60-year-old woman, reactive for anti-HEV and HAV, ALT level of 349 IU/l, and an 80-year-old man, reactive for anti-HEV and HBV, ALT level of 301 IU/l. The overall age of HSP patients from the was 41.0 years (mean, SD 23.89 and median 42), with no statistical difference between sexes (Table 2).
Viral hepatitis antibodies detection according to sex, age and alanine aminotransferase levels.
ALT, alanine aminotransferase. HAV, hepatitis A virus. HBV, hepatitis B virus. HCV, hepatitis C virus.
Considering the overall investigation of viral hepatitis agents in the 200 patients from HSP, there were 52 (26%) positive samples for acute A, B, and C hepatitis viruses and 4% for HEV.
To the best of our knowledge, this is the first study in Brazil that evaluated the diagnosis of HEV infection in patients looking for care at emergency rooms with increased ALT levels, as investigated in other international studies.11-13
The general detection of viral hepatitis in patients with elevated ALT levels varies according to the phase of the disease and the detection method employed. In this study, patients in the acute phase of HEV infection (the 16 patients with RT-qPCR positive) presented increased ALT levels up 12 times higher (200 IU/L) than the upper limit of normal (ULN), whereas patients reactive for antibodies against any of the analyzed hepatitis viruses had ALT levels 60 times the ULN. Therefore, liver damage could be minimized by implementing molecular diagnostic tests in the hospital routine. Other epidemiological studies focusing on changes in ALT levels in a larger number of patients are needed to better understand the real frequency of viral hepatitis in this group of patients presenting at the emergency room. It seems important to conduct additional studies using sensitive laboratory methodologies in other regions of Brazil that have very diverse epidemiological contexts. Although this study evaluated patients of low- to middle-income class of Sao Paulo city, the results could probably be different in other countries.
The use of molecular or serological HEV tests in patients with increased ALT levels could help disrupt the chain of transmission and prevent the spread of the disease.