Strongyloidiasis is a helminthiasis of neglected condition that has no gold standard parasitological diagnosis due to the intermittent release of larvae in feces. This study aimed to use an scFv (single chain variable fragment) obtained by Phage Display, previously validated to detect immune complexes in serum samples from individuals infected with Strongyloides stercoralis by enzyme-linked immunosorbent assay (ELISA). Now the ability of scFv to detect the immune complexes was verified by immunofluorescence, flow cytometry using magnetic beads and surface plasmon resonance (SPR). As ELISA, the SPR, immunofluorescence and flow cytometry demonstrated the ability of scFv to detect immune complexes in sera from individuals with strongyloidiasis and discriminate them from sera of individuals with other parasitic diseases and healthy individuals. Besides de conventional ELISA, the novel approaches can also be promptly applied as auxiliary diagnostic tools to the existing parasitological method for accurate diagnosis of human strongyloidiasis.
Human strongyloidiasis is a helminth infection of worldwide distribution. Considering only acute infections, strongyloidiasis is one of the major intestinal infections in humans. An important feature of this parasitic disease occurs by the possibility of autoinfection in which large amount of infective larvae present in the intestine spread to other organs. This process is more frequent in infected individuals under immunosuppression.1–3
Several parasitological, immunological and molecular biology methods have been described for diagnosis of human strongyloidiasis. Parasitological methods are often used to identify rhabditoid larvae, but due to small and irregular release of larvae in the feces these methods have low sensitivity. Immunological methods have the advantage of showing high sensitivity compared to parasitological methods, besides being useful in the evaluation of host immune response and in seroepidemiological surveys.4,5
Due to the large number of potentially exposed people with subclinical infection or misdiagnosed, this parasitosis is presented as a neglected condition. Considering the magnitude of strongyloidiasis and the importance of early diagnosis, there is an urgent need to develop diagnostic methodologies that are innovative, because of the possibility of chronicity, hyperinfection and dissemination, which can be fatal in immunosuppressed individuals.6–8
The aim of this study was to further improve the ELISA assay for strongyloidiasis diagnosis, as a reference method, which is based on the detection of an immune complex with an scFv (single chain variable fragment). The scFv binds to a specific protein from Strongyloides sp., HSP60, and it was used to detect immunocomplexes in human sera. This serodiagnosis demonstrated 97.5% sensitivity and 98.81% specificity to Strongyloides stercoralis.9 Now three novel biotechnological approaches were introduced, surface plasmon resonance, immunofluorescence and flow cytometry that are discussed herein.
Material and methodsEthical approvalAll experimental procedures were performed in accordance with the ethical guidelines of the Brazilian Health Ministry, and approved in 2013 (protocol number 307.605) by the Research Ethics Committee from the Federal University of Uberlândia, Minas Gerais state, Brazil.
Serum samplesSerum samples were the same used previously,10 comprising 124 human sera which were pooled (10μL of each serum sample) and divided into three groups: Group 1 (P1) – 40 serum samples from individuals infected only with strongyloidiasis; Group 2 (P2) – 44 serum samples from individuals positive by single infection of other intestinal parasitic diseases including Ascaris lumbricoides (n=8), hookworm (n=7), Enterobius vermicularis (n=5), Trichuris trichiura (n=5), Schistosoma mansoni (n=4), Hymenolepis nana (n=4), Taenia sp. (n=6) and Giardia lamblia (n=5); Group 3 (P3) – 40 serum samples from healthy individuals, based on their clinical observation, without contact or previous infection by Strongyloides and three fecal samples tested negative.
Sandwich enzyme-linked immunosorbent assayHigh affinity microtiter plates (Nunc MaxiSorp™) were incubated with 50μL of scFv (10μg/mL) in carbonate bicarbonate buffer (0.06M, pH 9.6), overnight at 4°C. Plates were blocked with PBS/5%BSA. Pooled serum samples were diluted 1:50 in PBS/0.05% Tween 20 (PBST) (v/v) and 5% BSA (w/v) and added to the wells. Subsequently it was added a mouse anti-human IgG peroxidase conjugated diluted in PBST (1:10,000). In the steps of blocking, sera and conjugated were incubated for 45min at 37°C and between each step it was performed three washes with PBST for 5min. The reaction was revealed by adding orthophenylenediamine (OPD) diluted in 0.1M citrate-phosphate (pH 5.0) and 30% H2O2. Plates were incubated for 15min at room temperature, and reactions were stopped by adding 2N H2SO4. The optical densities (OD) were determined at 492nm in an ELISA reader (Titertek Plus, Flow Laboratories, USA).10
Surface plasmon resonanceA gold electrode was prepared by using a cleaning solution, comprising of 3:1 H2SO4 PA/H2O2 PA, to remove all possible contaminants. All steps were performed using a surface plasmon resonance equipment (Metrohm, AUTOLAB B.V., Utrecht, Netherlands). The electrode was coupled to the equipment, and after two washes in 0.1M phosphate buffer (pH 7.4), 100μL of scFv (10μg/mL) was added. The electrode was blocked with 1% casein and pooled sera (1:10) was added. The whole process was carried out at 35°C. The three steps: scFv incorporation, blocking, and sera addition, were performed for 30min; and in between all steps, two washes were performed in 0.1M phosphate buffer (pH 7.4) followed by 30min in the same buffer until obtaining the baseline.
Immunofluorescence and flow cytometryMagnetic epoxy beads (Dynabeads M270 Epoxy, Invitrogen, Carlsbad, CA, USA) were washed three times in 0.1M phosphate buffer (pH 7.4) using a magnetic apparatus DynaMag-2. One hundred microliters of scFv (750μg/mL) and 3:1 ammonium sulphate solution 3M/1.2M phosphate buffer (pH 7.4) were added to 10μL of magnetic beads (2×107beads), and further incubated overnight at 4°C under stirring. Beads were blocked with PBS/5%BSA. One hundred microliters of pooled sera (1:50) were added and then 100μL of anti-human IgG/FITC (1:200) both diluted in PBS/5%BSA. In the steps of blocking sera and conjugated were incubated for 45min at 18°C and between each step it was performed three washes with PBST for 5min. Beads were analyzed in a fluorescence microscope (AMG EVOS fl, Cell Imaging System, Thermo Fisher Scientific Inc., Waltham, MA, USA) followed by flow cytometry (10,000 events/sample) (Accury™ C6, BD Biosciences, San Diego, CA, USA).
Statistical analysisStatistical analysis was performed using GraphPad Prism 5.0 (GraphPad Software Inc., San Diego, USA). Analysis of variance (ANOVA) followed by the Tukey post hoc test was used to determine differences among groups. Values were considered statistically significant when P<0.05.
ResultsEnzyme-linked immunosorbent assay (ELISA), surface plasmon resonance (SPR) and immunofluorescence/flow cytometry methods were conducted in order to verify the ability of scFv to detect immune complexes in sera from positive individuals with strongyloidiasis and to discriminate from those positive with other parasitic diseases and from healthy individuals. Similar to ELISA, SPR, immunofluorescence (IF)/flow cytometry (FC) approaches showed to be highly effective to detect S. stercoralis infection by using scFv and human sera. General features of the methods are shown in Table 1. The SPR technique demanded less time to be done and the turnaround time (2h and 30min) compared to the IF/FC and ELISA (3h and 15min). Furthermore, it is important to emphasize that both methods require the immobilization of the scFv for 18h prior to assays.
General features of methods to verify the ability of scFv to detect immune complexes in human sera from individuals infected by Strongyloides stercoralis.
| ELISA | IF/flow cytometry | SPR | |
|---|---|---|---|
| Material for assay | Microtiter plates | Magnetic beads | Gold electrode |
| scFv immobilization | 18h | 18h | 30min |
| Blocking | 45min | 45min | 30min |
| Serum samples | 45min | 45min | 30min |
| Detection antibody | 45min | 45min | – |
| Total time for washing | 1h | 1h | 1h 30min |
| Total time without immobilization | 3h 15min | 3h 15min | 2h 30min |
ELISA, enzyme-linked immunosorbent assay; IF, immunofluorescence; SPR, surface plasmon resonance.
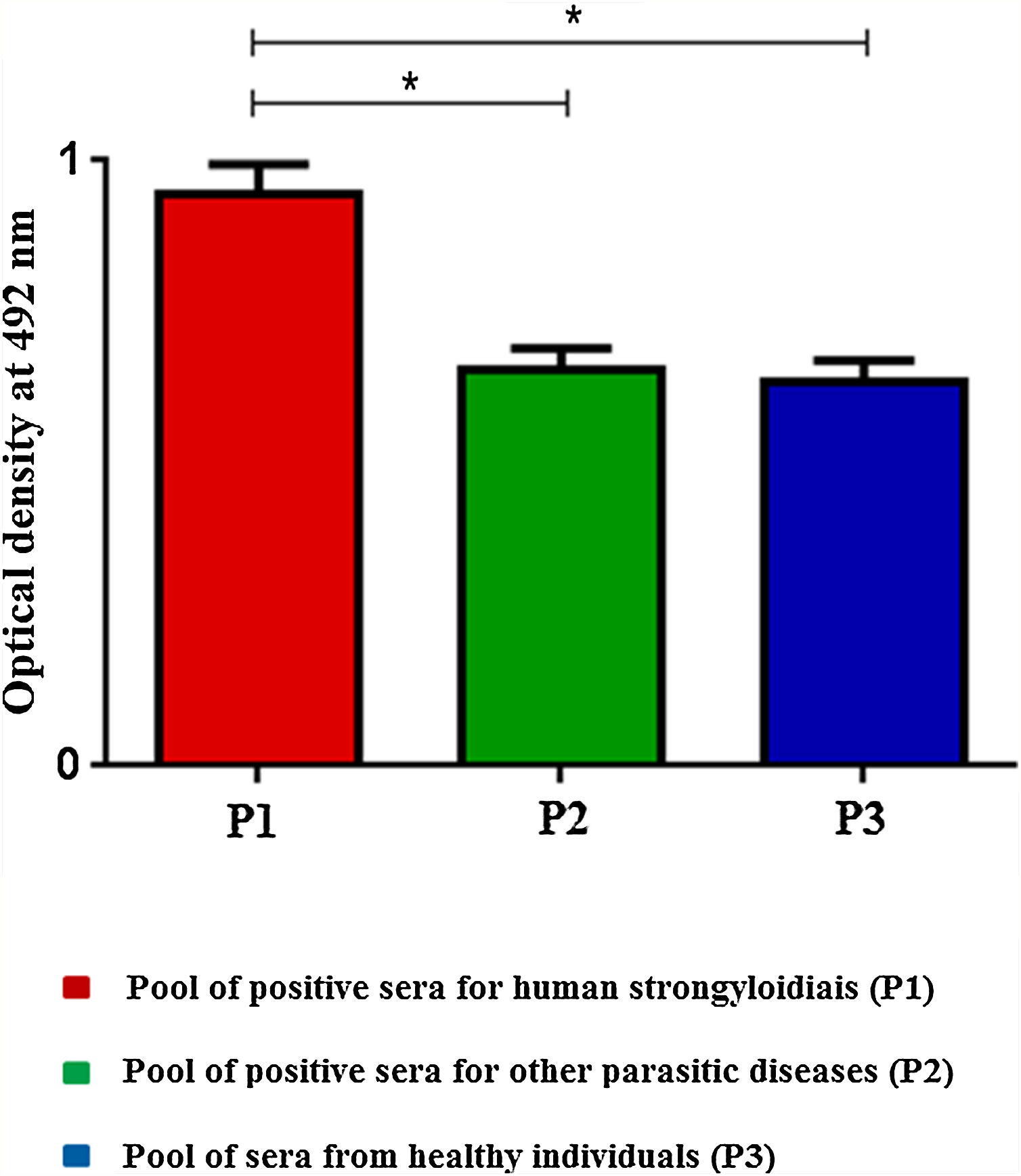
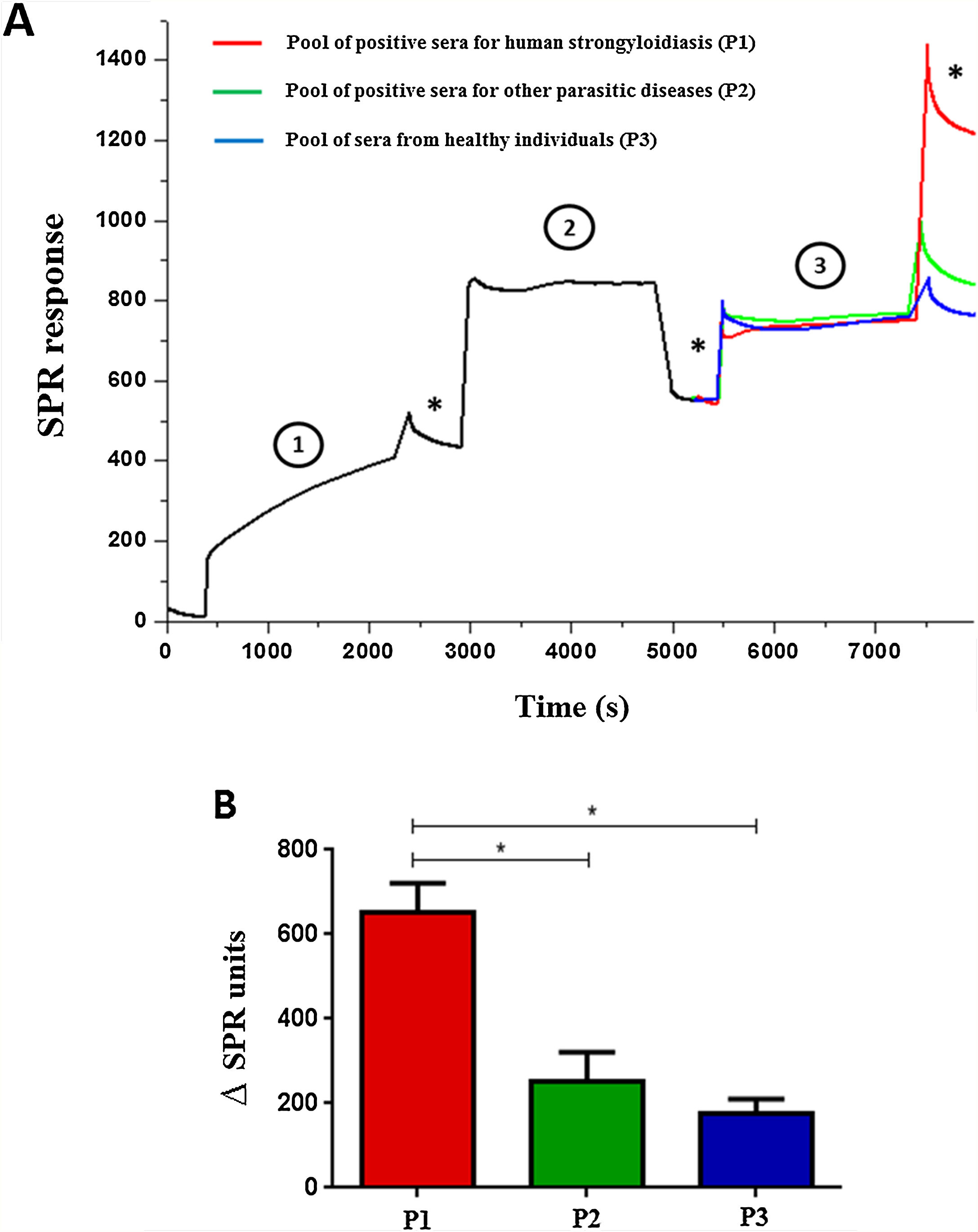
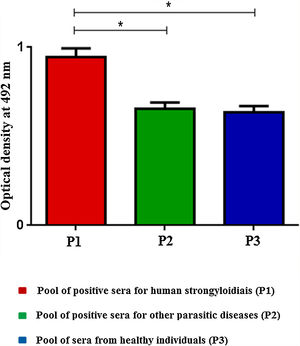
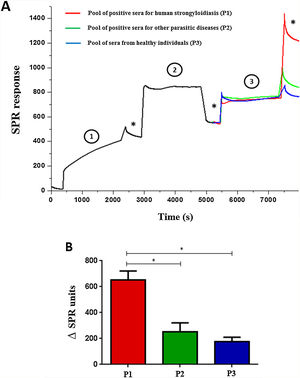
ELISA was performed by scFv immobilization in microtiter plates to detect immune complexes and differences on optical densities (492nm) were obtained according to the pool of sera (Fig. 1). The SPR showed high efficiency during the whole process; from immobilization of the scFv to the gold electrode (1), blocking (2), incorporation of sera (3), and washings (*) steps (Fig. 2A). The end result of the process demonstrated the significant difference in the SPR response in accordance with the pool of sera added (Fig. 2B).
Surface Plasmon Resonance using scFv to detect immune complexes in pooled human sera (A) and variation between the end result and early immobilization of each pool of sera (B). The gold electrode was added of scFv molecules (1) blocked with 1% casein (2) and sera pool samples were added (3). (*) Washing steps in phosphate buffer. Data are expressed as mean±standard deviation (n=2) and are representative of two independent experiments with similar results. *P<0.05.
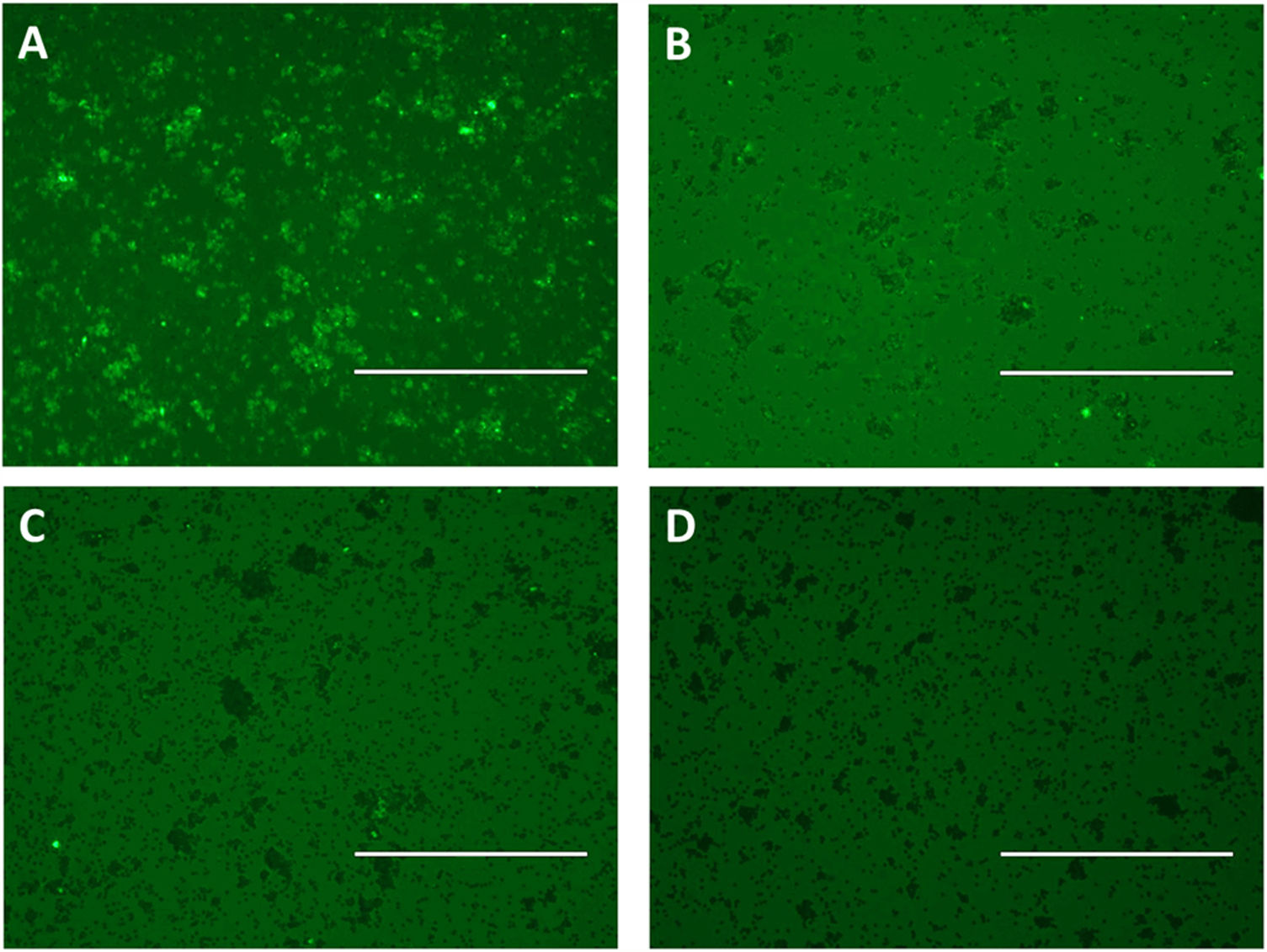
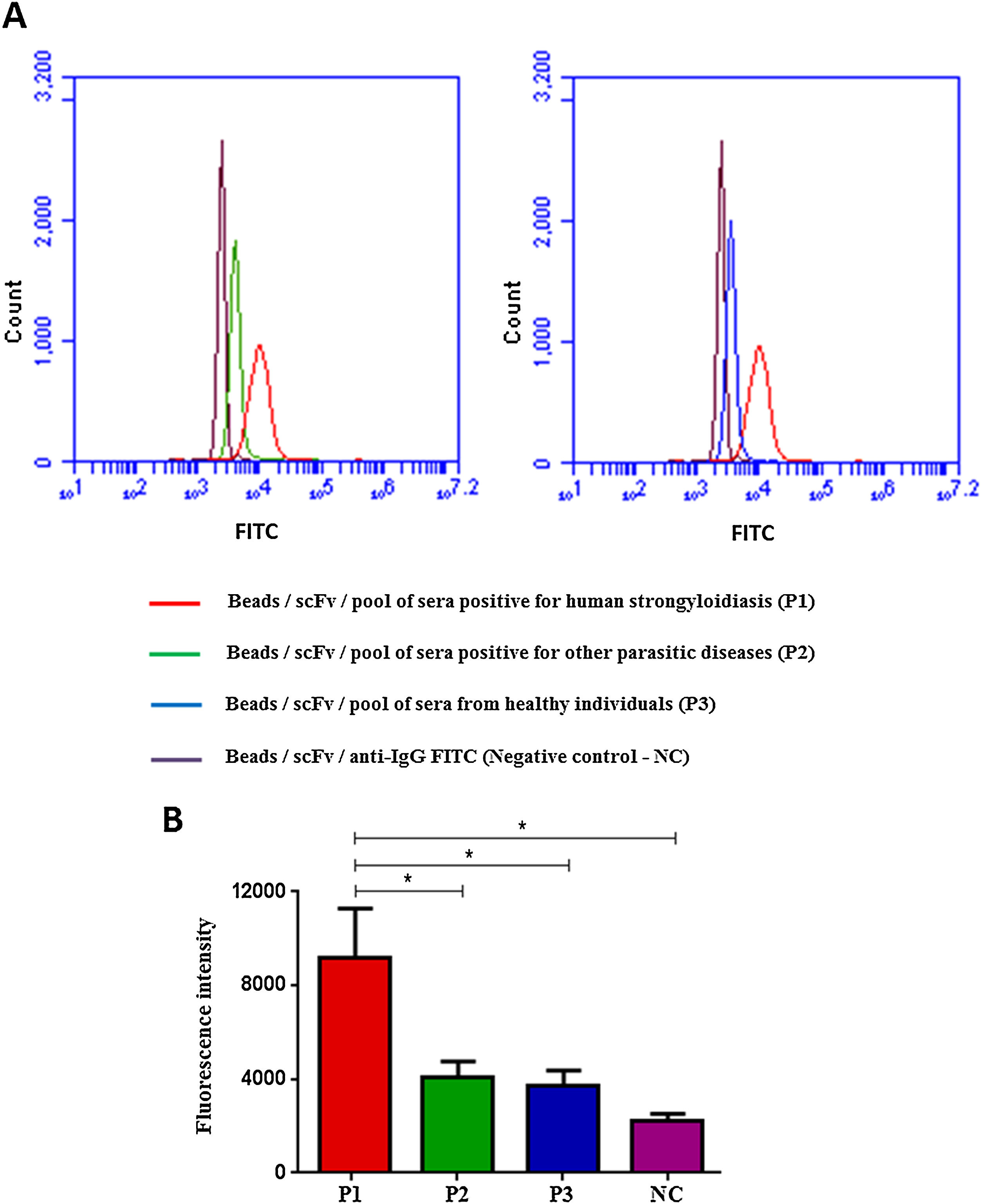
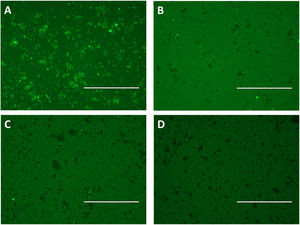
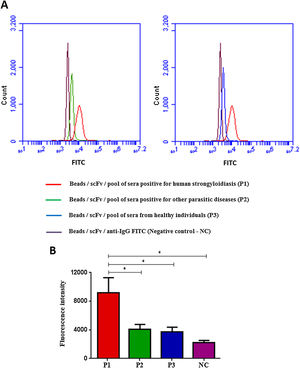
Flow cytometry was preceded by immunofluorescence, which showed that beads were fluorescent after immobilization of scFv in the beads and according to the pool of sera used: positive for strongyloidiasis (Fig. 3A), positive for other parasites (Fig. 3B) and healthy individuals (Fig. 3C). As a negative control beads were coupled with scFv and added anti-human IgG/FITC to the beads (Fig. 3D). Subsequently, this result could be seen, confirmed and counted by flow cytometry (Fig. 4A). It was observed significant differences in the fluorescence intensity of the beads according to the pool of sera (Fig. 4B).
Immune complexes detection by immunofluorescence using epoxy beads coupled by scFv and pooled human sera positive for strongyloidiasis (A) and other parasites (B) and pooled sera from healthy individuals (C), labeled with anti-human IgG/FITC. As a negative control were used scFv coupled beads and added anti-human IgG/FITC (D). Bar: 200μm.
Flow Cytometry with beads coupled with scFv and added of sera pool (A) and fluorescence intensity of each test (B), labeled with anti-human IgG/FITC. Data are expressed as mean±standard deviation (n=2) and are representative of two independent experiments with similar results. Count: 10,000 events; CN: negative control. *P<0.05.
Human strongyloidiasis is a neglected condition because it has underestimated prevalence data mainly due to the parasitological methods frequently used to detect larvae.5 Among immunological methods routinely applied, the ELISA technique has great advantage over other immunological methods due to the possibility of detecting circulating immune complexes, which is indicative of active infection.10–14
The development of functional probes, such as an scFv (single-chain variable fragments), has allowed the selection of high affinity molecules through Phage Display against various antigens of interest. These antibody fragments may represent an effective tool in the development of more specific diagnostic methods.15 In this study, we have used a previously selected specific scFv against the heat shock protein 60 (HSP60 antigen) from S. venezuelensis, and successfully applied in the immunodiagnosis of human strongyloidiasis.
The ability of the scFv to detect immune complexes (HSP60+IgG) in sera from individuals with strongyloidiasis was analyzed by surface plasmon resonance (SPR), Immunofluorescence/flow cytometry and compared to ELISA. In both new methods, the scFv molecule was able to detect immune complexes in a pooled sera of positive individuals for strongyloidiasis, and discriminated from individuals that were positive for other parasitic diseases and from healthy individuals.
Unlike ELISA, in which scFv is coated in microtiter plates, SPR used an scFv-coated gold electrode whereas the immunofluorescence/flow cytometry used epoxy-functionalized beads. SPR provided real-time visualization and demonstrated the efficiency of all stages of the process, from the immobilization of the scFv at the gold electrode, blocking step, samples incorporation, washings, and final result. Additionally, different from other techniques, SPR required less time to achieve diagnosis, and no need to use secondary antibodies for detection, which may be called label-free. Thus, the SPR technique presented as advantages the shorter turnaround time and practicality. The drawbacks of this technology are the requirement of well-trained human resource, medium to high cost equipment, and measurements can only be performed in multiple samples in high-end expensive equipment. However, this is the first demonstration that SPR can be used for human strongyloidiasis diagnosis. Only recently a study showed that this technique could be efficiently applied in serologic detection of IgM antibodies in dengue virus-infected individuals.16
There is no data in the literature that demonstrate the use of flow cytometry with epoxy beads to detect human strongyloidiasis. A method that uses beads coupled with protein A/G called LIPS (Luciferase Immunoprecipitation System), which analyzes the results in a luminometer has been applied in the detection of serum antibodies from individuals with strongyloidiasis and demonstrated high efficiency.17,18 But, this is the first study that applied flow cytometry to detect immune complexes in the human strongyloidiasis, which is an important feature since immune complexes represents a serum biomarker of active infection. Our flow cytometry (FC) method was preceded by analysis of beads in fluorescence microscope, which provided a preview of the result, according to the pool of sera used, which may also be considered a qualitative approach.
The establishment of a conventional ELISA to detect immune complexes for human strongyloidiasis diagnosis is of great public health importance worldwide, due to the possibility of disease chronicity and hyperinfection that are potentially fatal to humans. The development of complementary innovative approaches also offer new possibilities to improve Strongyloides sp. detection, and the description of three methods in this investigation; SPR, immunofluorescence and FC may become diagnostic choices that can be applied according to specific demand and resources settings. According to the present study, the immunofluorescence technique using magnetic beads may be more appropriate in low-resources laboratories, but an immunofluorescence microscope is still needed. In these places the dipstick technology may represent the best method to be used in the field. However, there is still no methodology that allows the detection of antigens or immune complexes such as the developed in the present study. All of them are used for IgG detection that may not be an indicative of active infection.
Conflicts of interestThe authors declare no conflicts of interest.
FundingThis work was supported by the Brazilian funding agencies, Conselho Nacional de Desenvolvimento Científico e Tecnológico – CNPq [grant numbers 302426/2012-4 and 490574/2010-6] – J. M. Costa-Cruz; CAPES [Rede Nanobiotec/Brasil Project N. 8], and Fundação de Amparo à Pesquisa do Estado de Minas Gerais – FAPEMIG [grant numbers CBB-PPM-00396-13 and the Excellence Center Program – PRONEX–APQ 02413-08] – L. R. Goulart.