This study aimed to investigate the serum levels of the cytokine TNF-α and its soluble receptors (sTNFR1 and sTNFR2) in patients with toxoplasmosis retinochoroidits (TR) and controls. 37 patients with TR and 30 subjects with positive serology for toxoplasmosis but without history and signs of uveitis were included in this study. Serum concentrations of TNF-α, sTNFR1, and sTNFR2 were determined by ELISA. Serum concentrations of TNF-α and sTNFR1 were similar in controls (mean ± SD median values; 56.57±141.96 and 504.37±163.87, respectively) and TR patients (mean ± SD values, 121.62±217.56 and 511.15±189.30, respectively). Serum concentrations of sTNFR2 were higher in the uveitis group when compared to the control group (respectively, mean ± SD values, 1734.84±379.32 and 1442.75±309.47; p=0.002). There was no association between the serum levels of the molecules and the time of first symptoms, severity of vitreous haze, size or localization of active lesions, levels of visual acuity, and presence of vasculitis. These results suggest that TR is associated with changes in the circulating levels of inflammatory biomarkers, but they are not correlated with local/ocular signs.
Toxoplasmosis is a common infection worldwide, but the disease is often asymptomatic in immunocompetent individuals. It is one of the most common causes of posterior uveitis in both immunocompetent and immunocompromised individuals.1 Toxoplasmic retinochoroiditis (TR) is the most common identifiable cause of posterior uveitis in many parts of the world, especially in Brazil.2
Few infected subjects develop ocular lesions, and it is uncertain why this happens. The intensity of the damage to the retina and choroid depends on the virulence of the infection and the associated inflammatory reaction.3,4 The response to antibiotic therapy in combination with corticosteroids and the clinical presentation vary significantly, with some patients presenting one episode of mild inflammation, and others having multiple recurrences of severe uveitis leading to loss of sight. The immune response is likely to play a role in determining the evolution of disease and possibly in the response to conventional therapy.5,6
TNF-α is a cytokine secreted in response to injury or infection by different cell types, and it coordinates early pro-inflammatory signals during innate immune response and is responsible for many of the associated systemic effects.7–9 TNF-α exerts its main effects by binding to two high-affinity cell surface receptors, TNFR1 (p55) and TNFR2 (p75).10 The extracellular portions of these receptors may constitute soluble forms (sTNFR1 and sTNFR2) and can be measured in the circulation.8 Since the soluble forms of TNF receptors can compete with the cell-associated TNF-receptors for TNF, it was suggested that they may function as inhibitors of TNF activity. Conversely, it has been shown that low concentrations of sTNFRs stabilize the activity of TNF-α by gradual cytokine release.11 Therefore sTNFRs may play a role as modulators of the biological activity of TNF-α. Therefore, measurement of circulating levels of the two sTNFRs may be useful to determine the overall production of TNF-α, and may be considered more reliable markers of inflammatory activity than TNF-α concentration itself.8,12,13 Since the production of sTNFRs is led by TNF-α, their concentrations may reflect TNF-α activity.
Many studies have documented that TNF-α is important for controlling resistance to acute14,15 and chronic16,17T. gondii infection. Experimental studies suggest that the TNF-α/TNFRs pathway may be beneficial for host protection against T. gondii.18,19 Immunity to T. gondii depends on TNFR1-mediated immune reactions,20 and experimental studies have demonstrated the crucial role of TNF-α for controlling intracerebral toxoplasmosis.16 In an animal model of ocular toxoplasmosis, treatment with anti-TNF-α antibodies resulted in worsening of ocular lesions, with the development of retinal necrosis and marked inflammation of the vitreous and underlying choroid.21
The aim of the present study was to evaluate serum levels of TNF-α and sTNFRs in TR patients compared to controls. It was also investigated whether their levels were associated with the clinical parameters of TR.
Material and methodsSubjectsThe study protocol adhered to the Declaration of Helsinki, and was approved by the local institutional review board. Patients were informed verbally and in writing of the potential benefits and risks of the study, and all patients signed an informed consent.
Adult subjects were recruited from the Uveitis Section of the Department of Ophthalmology of the Universidade Federal de Minas Gerais - Brazil. 37 patients with acute TR were invited to participate in this study (16 males, 21 females, mean age [± standard deviation, SD] 28.16±8.83 years). No subject enrolled in this study presented autoimmune or systemic infectious disease, or was under treatment for TR at the moment of the first evaluation. The diagnosis of TR was based on positive serological test for T. gondii (IgG) and typical ocular lesions in the retina (i.e. gray-white focus of retinal necrosis next to a pigmented retinal scar or in the other eye).1,2,22
All patients underwent a detailed ocular examination, including best-corrected visual acuity, applanation tonometry for intraocular pressure, slit lamp examination, fundus examination with 78-D lens, and indirect ophthalmoscope. The number and location of retinochoroidal lesions were documented for all patients by careful fundus drawings or photographs. The intensity of the inflammatory process was evaluated by the size of active retinochoroiditis lesions (graded by optic disc diameter [DD]), the presence of retinal vasculitis, and vitreous haze. Visual acuity (VA) was measured and expressed as the logarithm of the minimum angle of resolution (logMAR).
For comparison, 30 age- (mean age 30.37±6.46 years; p = 0.095; Mann-Whitney's U test) and gender-matched (13 males, 17 females; p = 0.994) adult subjects with no evidence of active ocular disease and with positive serological test (IgG antibody) for T. gondii were recruited for the control group. None had a history of uveitis, and each underwent a complete ocular examination (identical to the uveitis group) to rule out the presence of retinal scars suggestive of previous TR.
Blood sampling and biochemical measurementsIn all subjects, blood was collected aseptically after the ocular examination. Patients were with active disease and had not yet received any treatment. Serum was prepared and stored at -70°C. Serum levels were tested according to the procedures supplied by the manufacturer, using sandwich enzyme-linked immunosorbent assays (ELISA) kits for TNF-α, sTNFR1, and sTNFR2 (DuoSet, R&D Systems – Minneapolis, Minn.,USA), as routinely performed in this laboratory. All samples were assayed in duplicate. The values are presented in pg/mL and represent the mean result of the two measures. The variance between measures was under 10%.
Statistical analysisStatistical analysis and the graphs were performed using the Statistical Package for Social Sciences (SPSS) for Windows (16.0; SPSS, Inc., Chicago) and GraphPad Prism 4.00, respectively. All variables were tested for normality of distribution by means of the Kolmogorov-Smirnov test. Only the variable age showed normal distribution. Non-parametric analysis was performed using the Mann-Whitney's U test for comparison of unpaired data from two groups and the Willcoxon test for comparison of paired data. Differences between three groups were evaluated using the Kruskall-Wallis test. Correlation analysis between age and cytokine levels was performed using Spearman's correlation coefficient. Pearson's test was used for comparison of categorical data (e.g. gender). Statistical significance was set at p<0.05.
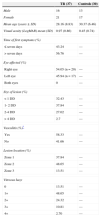
ResultsThirty-seven patients and 30 healthy age- and gender-matched controls were included in this study. There was significantly diminished visual acuity (VA – LogMAR) in the TR group: means of 0.97 and 0.45, in the TR and control groups, respectively (p=0.001). The main demographic and clinical features of patients with TR are presented in Table 1.
Clinical and demographic characteristics of patients with toxoplasmic retinochoroiditis (TR) and controls.
| TR (37) | Controls (30) | |
|---|---|---|
| Male | 16 | 13 |
| Female | 21 | 17 |
| Mean age (years ± SD) | 28.16 (8.83) | 30.37 (6.46) |
| Visual acuity (LogMAR) mean (SD) | 0.97 (0.86) | 0.45 (0.74) |
| Time of first symptoms (%) | ||
| ≤ seven days | 43.24 | — |
| > seven days | 56.76 | — |
| Eye affected (%) | ||
| Right eye | 54.05 (n = 20) | — |
| Left eye | 45.94 (n = 17) | — |
| Both eyes | 0 | — |
| Size of lesion (%) | ||
| <1 DD | 32.43 | — |
| 1- 2 DD | 37.84 | — |
| 2-4 DD | 27.02 | — |
| > 4 DD | 2.7 | — |
| Vasculitis (%)* | ||
| Yes | 58.33 | — |
| No | 41.66 | — |
| Lesion location (%) | ||
| Zone 1 | 37.84 | — |
| Zone 2 | 48.65 | — |
| Zone 3 | 13.51 | — |
| Vitreous haze | ||
| 0 | 13.51 | — |
| 1+ | 48.65 | — |
| 2+ | 24.32 | — |
| 3+ | 10.81 | — |
| 4+ | 2.70 | — |
There were no significant differences in serum concentrations of TNF-α and sTNFR1 between TR patients and controls. In contrast, significantly increased levels of sTNFR2 were found in TR patients (Table 2).
Serum levels (pg/mL) of TNF-α, sTNFR1 and sTNFR2 in toxoplasmic retinochoroidits (TR) patients and controls.
| Toxoplasmic retinochoroiditis | Controls | p | |||
|---|---|---|---|---|---|
| Mean ± SD | Median (interquartile range) | Mean ± SD | Median (interquartile range) | ||
| TNF-α | 121.62±217.56 | 11.45 (0-146.33) | 56.57±141.96 | 4.71 (0-37.70) | 0.258 |
| sTNFR1 | 511.15±189.30 | 452.67 (386.45-587.09) | 504.37±163.87 | 445.16 (388.15-638.01) | 0.99 |
| sTNFR2 | 1734.84±379.32 | 1699.51 (1411.08-2017.30) | 1442.75±309.47 | 142.79 (115.82-162.98) | 0.002 |
p-value: Mann-Whitney's U test; SD, standard deviation; 25-75%: quartiles 25 and 75.
There was no correlation among TNF-α, TNFR1, and TNFR2 even when controlling for TR. Correlation analyses also failed to establish any relationship between serum levels of the molecules and the time of first symptoms, severity of vitreous haze, size or localization of active lesions, levels of visual acuity, and presence of vasculitis.
DiscussionThere is a great controversy regarding which factors might be responsible for the occurrence or recurrence of TR. Among the proposed factors are the strains of parasites, hormonal changes, and the production of some molecules that participate in the immune system, such as cytokines.1,5
A recent study suggested that the chemokine CXCL8 can participate in the inflammatory process of TR and may be a useful marker for patient follow-up.23 It has also been suggested that polymorphisms of the genes responsible for the production of cytokines could predispose the occurrence and/or recurrence of TR. A study in humans found that the genotypes related to a low production of IL-10 may be associated with the occurrence of TR.24 Another study suggested that genotypes related with a high production of IL-1α may be associated with TR recurrence.25
To the best of the authors’ knowledge, this is the first study to assess the serum levels of TNF-α and sTNFRs in patients with TR and to correlate these levels with clinical parameters. The present results demonstrated increased levels of sTNFR2 in serum of patients with TR in comparison with controls, but no differences in TNF-α and sTNFR1 levels were found. The meaning of this change in circulating levels of sTNFR2 in TR is uncertain. This result may represent either systemic activation of inflammatory cascades or endocrine signaling at distance.
The protective role of cytokines such as TNF-α in TR has been demonstrated in animal models.21 In humans, few studies have investigated circulating levels of TNF-α, sTNFR1, and sTNFR2 in infectious uveitis. Takase et al.26 did not find any significant difference between the serum and aqueous humor levels of TNF-α in patients with anterior herpes simplex uveitis, acute retinal necrosis, and Vogt-Koyanagi-Harada uveitis. Yamamoto et al.27 analyzed blood samples of patients with acquired ocular toxoplasmosis and congenital ocular disease. They found higher levels of TNF-α in patients with acquired disease when compared with control subjects, but the difference was not statistically significant. Lahmar et al. (2009) analyzed the levels of TNF-α among other cytokines in aqueous humor of patients with TR and another infectious uveitis, finding similar levels of TNF-α in both conditions.28 The levels of cytokines in aqueous humor did not correlate with any demographic or clinical variables (including degree of uveal inflammation and etiology of the infection, i.e., congenital or primary acquired). Interestingly, the titers of TNF-α were significantly higher in aqueous humor in comparison with serum, suggesting that the cytokine is cleared more rapidly in this compartment.28 These findings of unaltered serum levels of TNF-α in infectious uveitis are in agreement with the present study. However, significantly increased serum levels of sTNFR2 in patients with TR were demonstrated in comparison with that of controls. As previous studies have suggested that sTNFR1 and sTNFR2 are the natural homeostatic regulators of the TNF-α11,29 and seem to be reliable markers of TNF-α activity8,12,13, the present finding of elevated sTNFR2 suggests the involvement of TNF-α in human TR.
The distinct behavior of sTNFR1 and sTNFR2 in TR is not unique and has been reported in other infectious diseases.8,12 Interestingly, a previous experimental study highlighted the role of TNFR1, but not TNFR2, in murine toxoplasmosis.14 Indeed TNFR1 and TNFR2 have different mechanisms of regulation.9 Inflammatory stimuli such as TNF-α and IL-1β increase the expression of TNFR2 through transcriptional activation, whereas TNFR1 is more commonly down-regulated by these same stimuli.30,31 It is worth mentioning one study which undermined the role of TNFR-mediated signaling in murine toxoplasmosis, indicating that interferon-receptor-mediated signaling is more relevant for the activation of cerebral blood vessel endothelial cells and microglia.32
In conclusion, the present results suggest that TR is associated with changes in the circulating levels of inflammatory biomarkers (sTNFR2), but they are not correlated with local/ocular signs. Additional studies involving repeated measurements with the same patients may elucidate a potential role for serum levels of sTNFRs as biomarkers of development and progression of TR.
Conflict of interestAll authors declare to have no conflict of interest.
This work was funded by the Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq, Brazil) and the Fundação de Amparo à Pesquisa de Minas Gerais (Fapemig, Brazil). These funding agencies had no role in the study design, collection, analysis, and interpretation of data; nor in the writing of the report; nor in the decision to submit the article for publication.