Treatment of multidrug-resistant Gram-positive infections caused by Staphylococcus aureus remains as a clinical challenge due to emergence of new resistance mechanisms. Tedizolid is a next-generation oxazolidinone, recently approved for skin and soft tissues infections. We conducted a study to determine in vitro susceptibility to vancomycin, daptomycin, linezolid and tedizolid in MRSA clinical isolates from adult patients with skin and soft tissue infections.
Material and methodsMethicillin-resistant S. aureus isolates were collected in three tertiary-care hospitals of Medellin, Colombia, from February 2008 to June 2010 as part of a previous study. Clinical characteristics were assessed by medical records and MIC values were determined by Epsilometer test. Genotypic analysis included spa typing, MLST, and SCCmec typing.
ResultsA total of 150 MRSA isolates were evaluated and tedizolid MIC values obtained showed higher in vitro activity than other antimicrobials, with MIC values ranging from 0.13μg/mL to 0.75μg/mL and lower values of MIC50 and MIC90 (0.38μg/mL and 0.5μg/mL). In contrast, vancomycin and linezolid had higher MIC values, which ranged from 0.5μg/mL to 2.0μg/mL and from 0.38μg/mL to 4.0μg/mL, respectively. Tedizolid MICs were 2- to 5-fold lower than those of linezolid. Clinical characteristics showed high previous antimicrobial use and hospitalization history. The majority of the strains belong to the CC8 harboring the SCCmec IVc and were associated with the spa t1610 (29.33%, n=44).
ConclusionIn vitro effectiveness of tedizolid was superior for isolates from skin and soft tissue infections in comparison with the other antibiotics evaluated. The above added to its less toxicity, good bioavailability, daily dose and unnecessity of dosage adjustment, make tedizolid in a promising alternative for the treatment of infections caused by MRSA.
Staphylococcus aureus is one of the most important pathogens in humans, being responsable for various types of infections in the healthcare and community settings.1,2 Among of these, skin and soft tissue infections (SSTIs) play an important role, not only by their high frequency but also due to severe forms that affect deep tissues, causing serious implications as high morbidity rates and increased hospital stay and costs.1,3
This microorganism is characterized by its high capacity to adapt to antimicrobials by the acquisition of several resistance mechanisms.2–4 Particularly, the emergence of methicillin-resistance isolates (MRSA) has limited therapeutic options, because this resistance involves low affinity for all β-lactam antibiotics.5
Vancomycin has been considered an effective option for the treatment of skin and soft tissue infections caused by MRSA. However, the high burden of these infections in hospitals has led to overuse of this antibiotic which in turn has led to increased values of minimum inhibitory concentration (MIC), emergence of intermediate and total resistance to this antimicrobial and increased mortality.6–8
This situation underscores the need for new antimicrobial options to the treatment of infections caused by MRSA which respond to changing resistance patterns.8 Currently, the therapeutic options approved by Food and Drug Administration (FDA) for the SSTIs by MRSA include daptomycin, linezolid, and the recently introduced, tedizolid.9,10
Tedizolid is a novel oxazolidinone with high potential of action that is approximately four times more potent than linezolid.9,10 Although the mechanism of action is similar to other oxazolidinones, tedizolid has shown more advantages, as lower adverse effects over short courses of therapy and favorable pharmacokinetics.9–11 Tedizolid shows an increased activity against species of staphylococci and enterococci, including drug-resistant MRSA and vancomycin- and linezolid-resistant phenotypes.9–11
Constant changes in the epidemiology of infections caused by S. aureus reaffirm that this microorganism is a major threat for public health and highlights the need to evaluate therapeutic alternatives that allow for a better prognosis of these infections.
In Colombia, MRSA (both healthcare-associated and community-associated) has become a worrisome clinical problem, with a general frequency of 27%, reaching in some cities up to 50%.12 This situation brings about new challenges for the treatment of this infections and patient safety.
Therefore, the aim of this study was to determine in vitro susceptibility to vancomycin, daptomycin, linezolid and tedizolid of MRSA clinical isolates from patients with skin and soft tissue infections, collected in three tertiary-care hospitals of Medellin, Colombia.
Material and methodsBacterial isolatesMethicillin-resistant S. aureus isolates were collected in three tertiary-care hospitals of Medellin, Colombia, from February 2008 to June 2010, as part of a previous cross-sectional study.13–15 Hospitals A and B are hospitals with high level of complexity with 754 and 286 beds, respectively, which provide services in all medical specialties; whereas hospital C is a 140-bed cardiology hospital.
Clinical and epidemiological informationClinical and epidemiological data were obtained from medical records. Information included demographic aspects, medical history, comorbidities, length of hospital stay and outcomes at discharge. Infections were classified as community-associated (CA-MRSA) or healthcare-associated (HA-MRSA), according to definitions established by the CDC.16
The research protocol was approved by the Bioethics Committee for human Research at Universidad de Antioquia (CBEIH_SIU-approval No. 0841150).
Strains and antibiotic susceptibilityThe identification of S. aureus was conducted by standard laboratory methods based on colony morphology in sheep blood agar and phenotyping methods such as catalase and coagulase. Antibiotic susceptibilities of S. aureus isolates were assessed in accordance with Clinical Laboratory Standards Institute guidelines (CLSI, 2009) using a VITEK-2® instrument (bioMérieux Lyon, France). The Antibiotics evaluated included clindamycin, erythromycin, gentamicin, linezolid, moxifloxacin, oxacillin, rifampin, tetracycline, tigecycline, trimethoprim-sulfamethoxazole and vancomycin.
Evaluation of the susceptibility through Epsilometer testThe MICs to vancomycin, daptomycin, linezolid and tedizolid were determined using the Epsilometer test method (E-test) according to the manufacturer's instructions (Etest® bioMérieux and Liofilchem® MIC Test Strip). MIC50 and MIC90, that correspond respectively to the 50% and the 90% of strains inhibited by a specific concentration of each antibiotic were obtained. The MIC breakpoints were determined according to the criteria defined by CLSI, 2016 as follows: for vancomycin, values less than or equal to 2μg/ml, between 4 and 8μg/ml, or greater than or equal to 16μg/ml were considered susceptible, intermediate and resistant, respectively; for daptomycin, these values were less than or equal to 1μg/ml for susceptibility and higher values were deemed as non-susceptible; for linezolid, values less than or equal to 4μg/ml were deemed susceptible and greater than or equal to 8μg/ml resistant; finally, for tedizolid, values less than or equal to 0.5μg/ml were considered susceptible, 1μg/ml intermediate and values higher than or equal to 2μg/ml resistant. Strain ATCC 29213 of S. aureus was used as a control strain.
Molecular characterizationPresence of the species-specific nuc and femA genes, as well as the mecA gene (determinant of methicillin resistance) were verified by polymerase chain reaction (PCR) as previously described.17,18 Genes encoding staphylococcal enterotoxins (sea, seb, sec, sed, see), toxic shock syndrome toxin 1 (tst), and exfoliative toxins A and B (eta, etb) were detected by multiplex PCR. The lukS/F-PV genes encoding Panton-Valentine leukocidin (PVL), as well as the arcA gene from the USA300-associated arginine catabolic mobile element (ACME), were also assayed.18,19 Strain typing was performed using protein A typing (spa), multilocus sequence typing (MLST), and SCCmec typing.20–23
Statistical analysesComparisons of clinical characteristics were carried out between CA-MRSA- and HA-MRSA-infected patients after applying CDC criteria. Categorical variables were compared using chi-square test or Fisher's exact test and Mann–Whitney U tests for continuous variables. p-Values≤0.05 were considered statistically significant. Statistical analyses were performed using the software package Stata® v14.0.
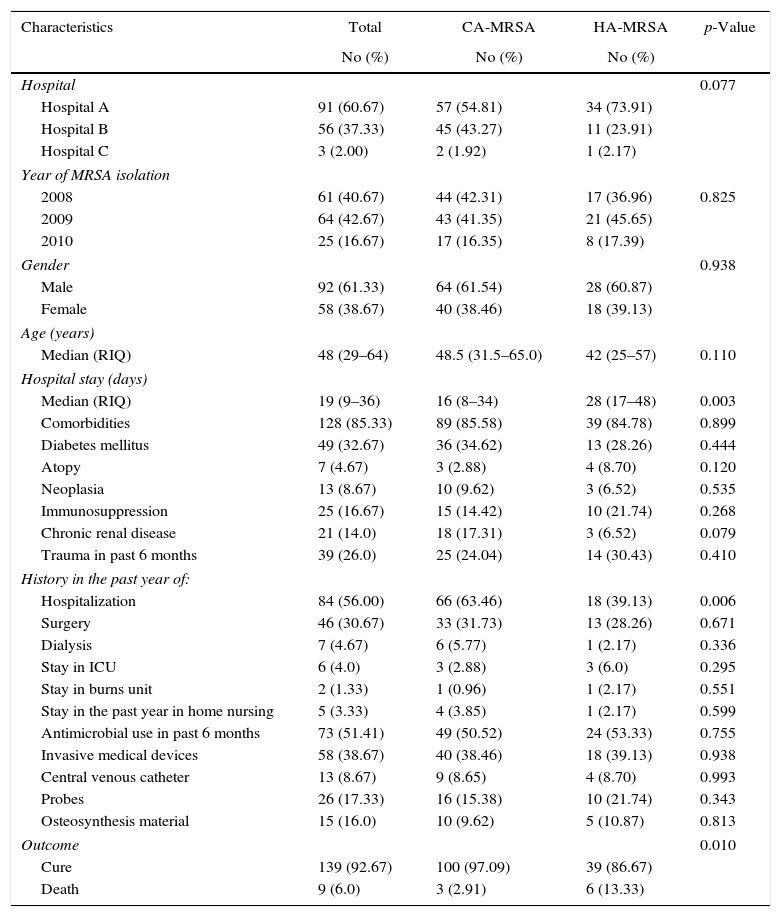
ResultsA total of 150 MRSA isolates from adult patients with skin and soft tissue infections were evaluated. The demographic and clinical characteristics of patients are summarized in Table 1. The majority of patients were male (61.33%, n=92), with an age range between 15 and 89 years old and hospitalized in hospital A (60.67%, n=91).
Clinical and epidemiological characteristics among MRSA infections.
| Characteristics | Total | CA-MRSA | HA-MRSA | p-Value |
|---|---|---|---|---|
| No (%) | No (%) | No (%) | ||
| Hospital | 0.077 | |||
| Hospital A | 91 (60.67) | 57 (54.81) | 34 (73.91) | |
| Hospital B | 56 (37.33) | 45 (43.27) | 11 (23.91) | |
| Hospital C | 3 (2.00) | 2 (1.92) | 1 (2.17) | |
| Year of MRSA isolation | ||||
| 2008 | 61 (40.67) | 44 (42.31) | 17 (36.96) | 0.825 |
| 2009 | 64 (42.67) | 43 (41.35) | 21 (45.65) | |
| 2010 | 25 (16.67) | 17 (16.35) | 8 (17.39) | |
| Gender | 0.938 | |||
| Male | 92 (61.33) | 64 (61.54) | 28 (60.87) | |
| Female | 58 (38.67) | 40 (38.46) | 18 (39.13) | |
| Age (years) | ||||
| Median (RIQ) | 48 (29–64) | 48.5 (31.5–65.0) | 42 (25–57) | 0.110 |
| Hospital stay (days) | ||||
| Median (RIQ) | 19 (9–36) | 16 (8–34) | 28 (17–48) | 0.003 |
| Comorbidities | 128 (85.33) | 89 (85.58) | 39 (84.78) | 0.899 |
| Diabetes mellitus | 49 (32.67) | 36 (34.62) | 13 (28.26) | 0.444 |
| Atopy | 7 (4.67) | 3 (2.88) | 4 (8.70) | 0.120 |
| Neoplasia | 13 (8.67) | 10 (9.62) | 3 (6.52) | 0.535 |
| Immunosuppression | 25 (16.67) | 15 (14.42) | 10 (21.74) | 0.268 |
| Chronic renal disease | 21 (14.0) | 18 (17.31) | 3 (6.52) | 0.079 |
| Trauma in past 6 months | 39 (26.0) | 25 (24.04) | 14 (30.43) | 0.410 |
| History in the past year of: | ||||
| Hospitalization | 84 (56.00) | 66 (63.46) | 18 (39.13) | 0.006 |
| Surgery | 46 (30.67) | 33 (31.73) | 13 (28.26) | 0.671 |
| Dialysis | 7 (4.67) | 6 (5.77) | 1 (2.17) | 0.336 |
| Stay in ICU | 6 (4.0) | 3 (2.88) | 3 (6.0) | 0.295 |
| Stay in burns unit | 2 (1.33) | 1 (0.96) | 1 (2.17) | 0.551 |
| Stay in the past year in home nursing | 5 (3.33) | 4 (3.85) | 1 (2.17) | 0.599 |
| Antimicrobial use in past 6 months | 73 (51.41) | 49 (50.52) | 24 (53.33) | 0.755 |
| Invasive medical devices | 58 (38.67) | 40 (38.46) | 18 (39.13) | 0.938 |
| Central venous catheter | 13 (8.67) | 9 (8.65) | 4 (8.70) | 0.993 |
| Probes | 26 (17.33) | 16 (15.38) | 10 (21.74) | 0.343 |
| Osteosynthesis material | 15 (16.0) | 10 (9.62) | 5 (10.87) | 0.813 |
| Outcome | 0.010 | |||
| Cure | 139 (92.67) | 100 (97.09) | 39 (86.67) | |
| Death | 9 (6.0) | 3 (2.91) | 6 (13.33) | |
Above 69% of infections were classified as community-associated according to CDC criteria after individual assessment of cases; the medical histories revealed frequent use of antibiotics (51.41%, n=73), comorbidities mainly diabetes mellitus (32.67%, n=49), and history in the past year of hospitalization and surgery (56%, n=84 and 30.67%, n=46, respectively). The most frequent outcome was cure (92.67%, n=139).
When comparing clinical characteristics among MRSA infections (CA-MRSA and HA-MRSA), three variables showed statistically significant differences: hospital stay (p=0.003), history of hospitalization (p=0.06) and outcome (p=0.010). Length of hospital stay was longer for patients with HA-MRSA infections, while previous hospitalization had a major tendency among CA-MRSA infections. The outcome death was more frequent among HA-MRSA group.
Molecular characterization of isolatesThe presence of nuc, femA and mecA genes were confirmed in all isolates. The most common clonal complex was CC8 (63.3%, n=95), followed by CC5 (31.3%, n=47). Furthermore, CC45 (1.3%), CC1 (0.7%) and CC30 (0.7%) were detected in low frequency.
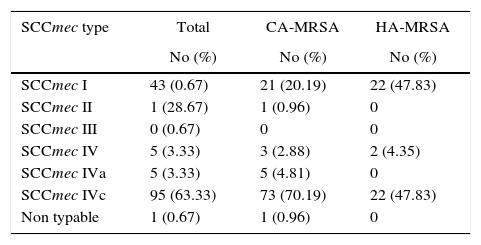
Regarding typing of chromosome cassette mec (SCCmec), a high percent of isolates classified as CA-MRSA and HA-MRSA harbored SCCmec type IVc (n=73, 70.19% and n=22, 47.83%, respectively), followed by SCCmec type I (n=21, 20.19% and n=22, 47.83%, respectively). The remaining SCCmec types were present at very low frequency (Table 2).
SCCmec types among to CA-MRSA and HA-MRSA isolates.
| SCCmec type | Total | CA-MRSA | HA-MRSA |
|---|---|---|---|
| No (%) | No (%) | No (%) | |
| SCCmec I | 43 (0.67) | 21 (20.19) | 22 (47.83) |
| SCCmec II | 1 (28.67) | 1 (0.96) | 0 |
| SCCmec III | 0 (0.67) | 0 | 0 |
| SCCmec IV | 5 (3.33) | 3 (2.88) | 2 (4.35) |
| SCCmec IVa | 5 (3.33) | 5 (4.81) | 0 |
| SCCmec IVc | 95 (63.33) | 73 (70.19) | 22 (47.83) |
| Non typable | 1 (0.67) | 1 (0.96) | 0 |
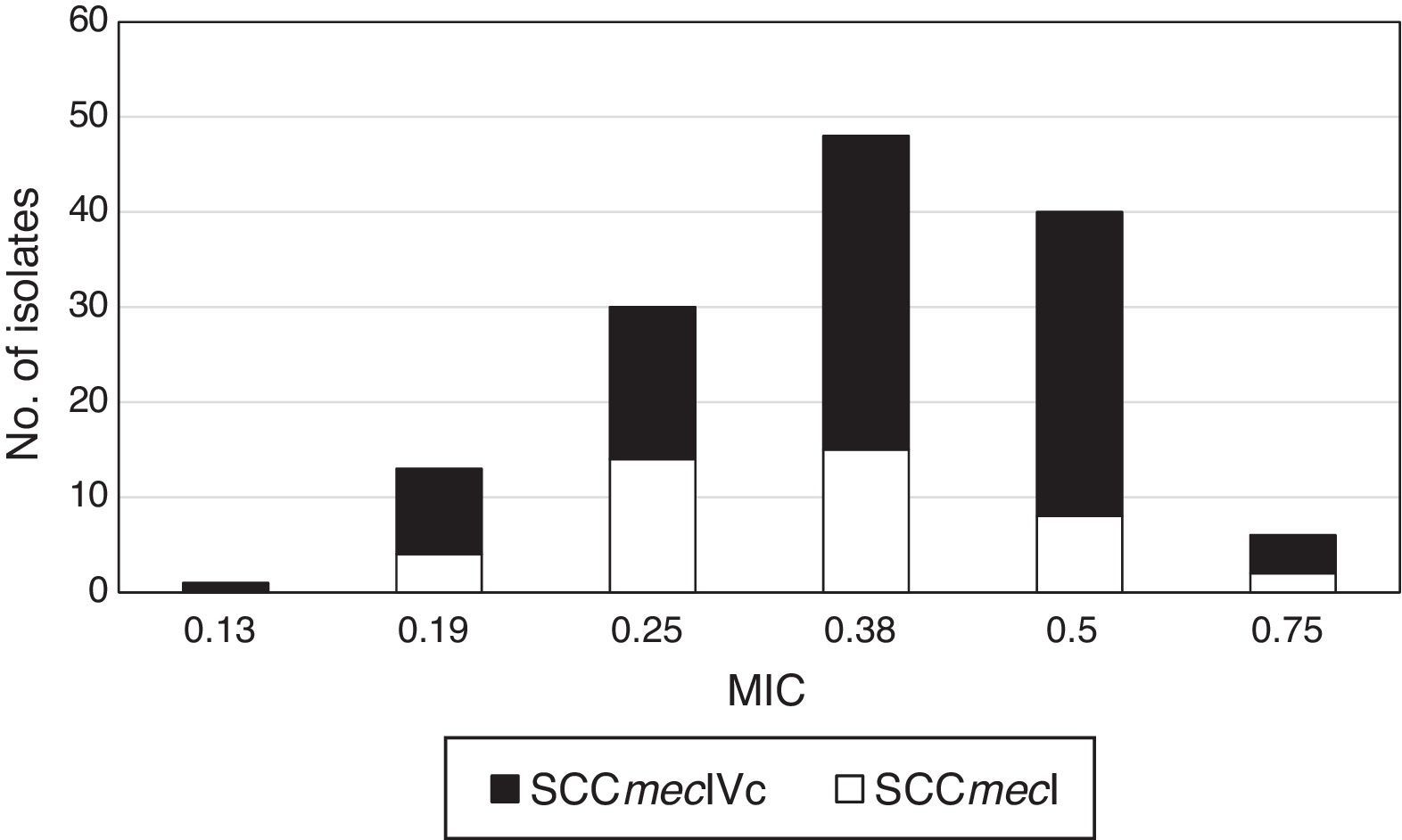
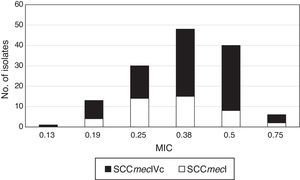
Typing of chromosome cassette mec according to tedizolid MIC showed that the most common was the SCCmec type IVc (63.3%, n=95) for all isolates, independently to tedizolid MIC, followed by the SCCmec type I (28.7%, n=43) (Fig. 1).
Several types of the protein A were found: t1610 (30.67%, n=46), t149 (16.67%, n=25), t008 (11.33%, n=17), and others as t024 (10%, n=15), t1311 (4%, n=6) and t7279 (4%, n=6).
The strains carrying the SCCmec type I belonged to the spa t149 and CC5. While the strains carrying SCCmec IVc belonged to spa types t1610, t008, and t024 of the CC8.
CC8 MRSA strains harboring SCCmec type IVc and belonged to the spa t1610 were more frequent (29.33%, n=44) and presented MICs values for tedizolid between 0.38μg/mL and 0.50μg/mL.
Regarding to virulence factors, the most common gene was lukS/F-PV (85.8%, n=97), followed by enterotoxin B (18.6%, n=21), toxic shock syndrome toxin (17.7%, n=20) and enterotoxin D (8.8%, n=10) genes. Enterotoxin A, C and E were found in only three isolates (0.9%).
Antimicrobial susceptibilityAmong the total of MRSA isolates, 43.33% (n=65) and 35.33% (n=53) were resistant to tetracycline and erythromycin, respectively. Resistance to clindamycin, gentamicin, moxifloxacin and rifampin, was less than 21%.
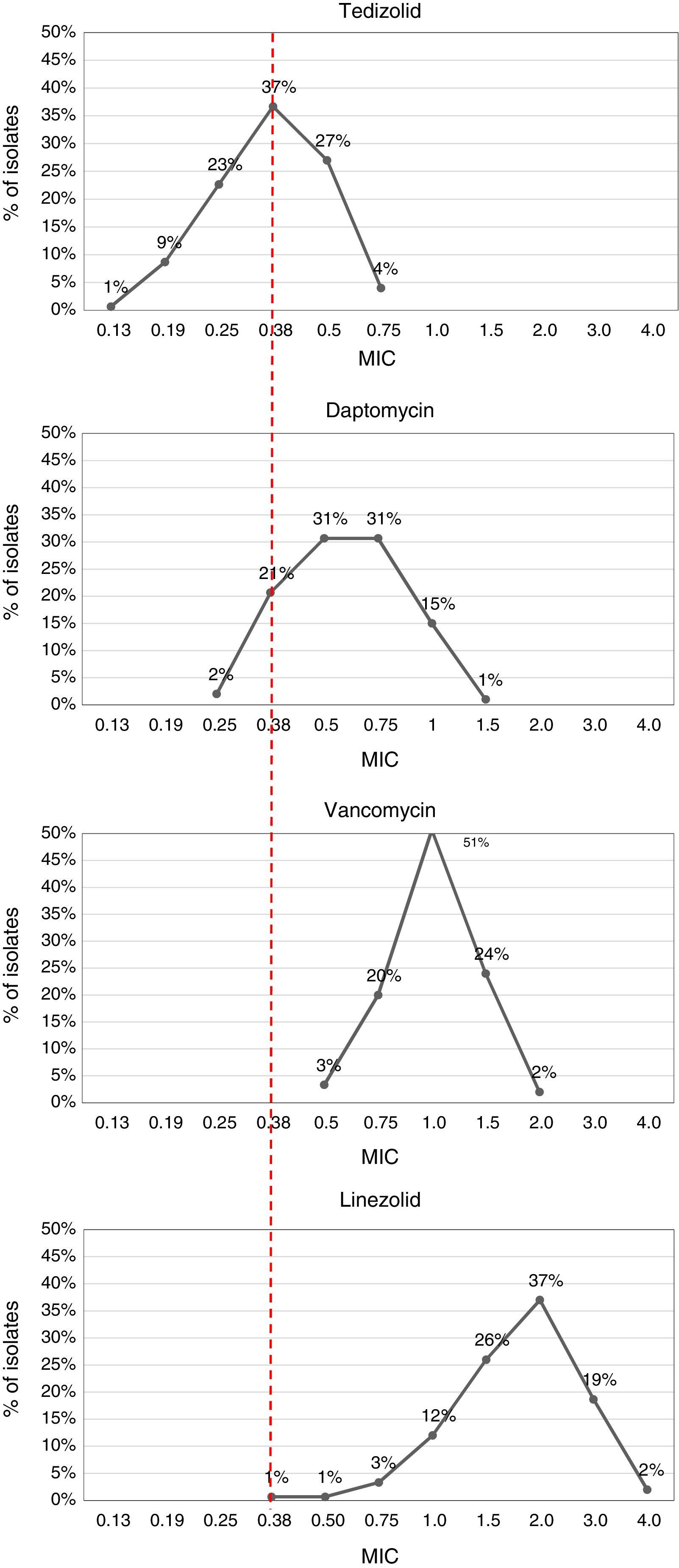
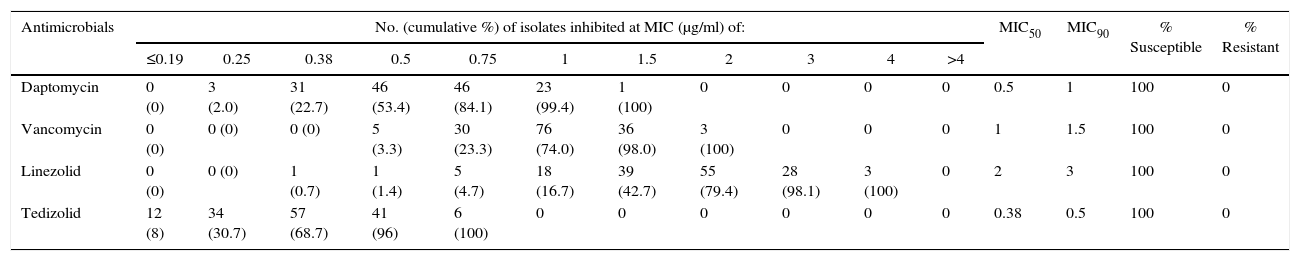
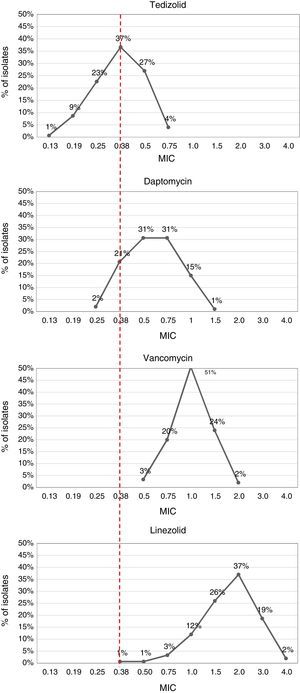
The MIC values obtained through E-test showed susceptibility to vancomycin, daptomycin, linezolid and tedizolid, except for one isolate with MIC=1.5μg/mL to daptomycin (Table 3). Tedizolid was more active than other antimicrobials, with MIC values ranging from 0.13μg/mL to 0.75μg/mL, and lower values of MIC50 and MIC90 (0.38 and 0.5, respectively). In contrast, vancomycin and linezolid had high MICs with values even above 1.5μg/mL (Table 3, Fig. 2).
Frequency of occurrence and cumulative percent distribution of MICs for selected drugs against S. aureus isolates.
| Antimicrobials | No. (cumulative %) of isolates inhibited at MIC (μg/ml) of: | MIC50 | MIC90 | % Susceptible | % Resistant | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ≤0.19 | 0.25 | 0.38 | 0.5 | 0.75 | 1 | 1.5 | 2 | 3 | 4 | >4 | |||||
| Daptomycin | 0 (0) | 3 (2.0) | 31 (22.7) | 46 (53.4) | 46 (84.1) | 23 (99.4) | 1 (100) | 0 | 0 | 0 | 0 | 0.5 | 1 | 100 | 0 |
| Vancomycin | 0 (0) | 0 (0) | 0 (0) | 5 (3.3) | 30 (23.3) | 76 (74.0) | 36 (98.0) | 3 (100) | 0 | 0 | 0 | 1 | 1.5 | 100 | 0 |
| Linezolid | 0 (0) | 0 (0) | 1 (0.7) | 1 (1.4) | 5 (4.7) | 18 (16.7) | 39 (42.7) | 55 (79.4) | 28 (98.1) | 3 (100) | 0 | 2 | 3 | 100 | 0 |
| Tedizolid | 12 (8) | 34 (30.7) | 57 (68.7) | 41 (96) | 6 (100) | 0 | 0 | 0 | 0 | 0 | 0 | 0.38 | 0.5 | 100 | 0 |
Most isolates showed a tedizolid MIC of 0.38μg/mL (38%, n=57) and a linezolid MIC of 2μg/mL (36%, n=55). Interestingly, 18.7% (n=28) and 2% (n=3) of the isolates, presented MICs values of 3μg/mL and 4μg/mL to linezolid, respectively. Additionally, tedizolid MICs values were 3- to 5-fold lower than those of linezolid (Fig. 2).
The MICs values for the S. aureus ATCC 29213 control strain, falling within the range for the quality control recommended by CLSI (0.5–2μg/mL for vancomycin, 0.12–1μg/mL for daptomycin, 0.5–2μg/mL for linezolid, and 0.25–1μg/mL for tedizolid).
DiscussionTedizolid has been recently approved by the FDA for treatment of skin and soft tissue infections caused by Gram-positive bacteria, including MRSA.11 The importance of this antimicrobial is focused on the emergence of less susceptible or resistant MRSA strains to vancomycin (hVISA and VISA) and the discovery of a possible dissemination of cfr gene, that is horizontally transferable and confers resistance to linezolid.24
The results of this study evidenced that tedizolid was the most effective antibiotic compared with vancomycin, daptomycin and linezolid, showing lower MIC values although its resistance breakpoint is considerably lower than the other antimicrobials and being consistent with conclusions in other studies.25 This can be better appreciated with MIC50 and MIC90 values, that points to the effectiveness of the antibiotic and the behavior of susceptibility of MRSA isolates. MIC90 display important differences between the four antibiotics tested. Tedizolid was notably superior, with MIC90 values below 0.5μg/mL, while the MIC90 values were 1μg/mL, 1.5μg/mL, and 3μg/mL for daptomycin, vancomycin and linezolid, respectively.
Greater activity and a narrow range of tedizolid MICs (between 0.19 and 0.75μg/mL) has been reported in several studies across a variety of culture conditions and with reference and clinical isolates of S. aureus.26 A study carried out in South Korea reported a tedizolid MIC90 of 0.5μg/mL in Gram-positive isolates as MRSA, vancomycin-resistant enterococci, S. pneumoniae, S. pyogenes, S. agalactiae and Clostridium spp. and showed more potency of tedizolid than linezolid or vancomycin.27 Similarly, a survey study of 1063 Gram-positive and Gram-negative isolates from United States, Great Britain, France, Germany, and Australia revealed that tedizolid was 4–8 fold more potent against staphylococci than linezolid, with a MIC90 of 0.5μg/mL, and 2–8 fold more potent than vancomycin against these microorganisms.28 More recent studies have confirmed these results, which have shown tedizolid MICs 4–32 fold lower than those of linezolid in clinical isolates of linezolid-resistant staphylococci and enterococci,29 maintaining activity against isolates with reduced susceptibility to alternative agents such as vancomycin and daptomycin.25,30,31
Tedizolid and linezolid are both oxazolidinones and share many characteristics such as activity against Gram-positive bacteria, the same mechanism of action and high oral bioavailability. However, tedizolid has shown better in vitro activity compared to linezolid, as it can bind to additional targets sites in 23s rRNA which leads to activity against MRSA strains with linezolid resistance mediated by cfr gene mutation.32 Additionally, tedizolid is approximately 90% protein-bound and has a half-life near two-fold greater than linezolid, leading to only six days of therapy (tedizolid 200mg once daily for six days vs linezolid 600mg twice daily for 10 days) and not requiring dosage adjustment in patients with any renal or hepatic dysfunction.33–35 Furthermore, the toxicity and in vitro tedizolid resistance rates are lower than for other antimicrobials.36,37
In present study, high MICs values were observed for vancomycin and daptomycin, being beyond or in the upper limit of susceptibility. Most clinicians use vancomycin for empiric and definitive therapy for MRSA infections; however, recent reports have drawn attention to increased treatment failures at MIC 1μg/mL, although CLSI breakpoint defines the upper limit of susceptibility in 2μg/mL.8 Besides, clinical outcomes with vancomycin depend of bacterial load at site of infection, and higher rates of nephrotoxicity are associated with high-dose therapy.38
Daptomycin is an alternative to vancomycin for patients with MRSA infections, but some authors have recommended to use high doses to minimize the emergence of elevated MIC values, which has been associated with an increase in the vancomycin MIC and the heteroresistant phenotype (hVISA).39,40
Interestingly, in this study the activity of tedizolid was maintained in strains isolated from patients with different characteristics including an age range between 15 and 89 years old, different comorbidities and previous antimicrobial use. Likewise, the MIC values for tedizolid was similar in all isolates and the results were consistent in both community and health care-associated infections.
On the other hand, the most frequent genotype was CC8-t1610-SCCmecIVc, which is common in community-associated strains with Panton-Valentine leucocidin, against which tedizolid retained activity, too.41 These community-associated strains are being introduced to hospitals, replacing healthcare-associated strains, according to previous studies from Medellín.42
Efficacy, safety, and tolerability of tedizolid have been evaluated in Latino patients and tedizolid had presented comparable efficacy to linezolid, sustained clinical success rates, and lower abnormal platelet counts and gastrointestinal events, without warranting dose adjustment.43 Countries like Colombia, where MRSA has become a worrisome clinical problem, with a general frequency of 27%, reaching up to 50% in some cities,12 increased resistance rates and high antibiotic pressure generate additional cost to health system. Therapeutic alternatives like tedizolid are thus necessary to improve prognosis of infections caused by Gram-positive bacteria such as S. aureus.44
In conclusion, in vitro effectiveness of tedizolid was superior for isolates from skin and soft tissue infections in comparison with other therapy alternatives like vancomycin, daptomycin and even linezolid. The combination of less toxicity, good bioavailability, once daily dose and no requiring dosage adjustment makes tedizolid a promising alternative for the treatment of infections caused by S. aureus, including methicillin-resistant isolates.
FundingThis work was supported by BAYER S.A.
Conflicts of interestThe authors declare no conflicts of interest.