Infection with Human T cell Leukemia Virus type 1 can be associated with myelopathy/tropical spastic paraparesis (HAM/TSP) and other inflammatory diseases. Lymphocytes from about half of Human T cell Leukemia Virus type 1-infected subjects spontaneously proliferate in vitro, and how this phenomenon relates to symptomatic disease and viral burden is poorly understood.
ObjectiveTo evaluate T-cell proliferation in vitro among patients co-infected with Human T cell Leukemia Virus type 1/Hepatitis C Virus/Human Immunodeficiency Virus type 1.
Material and methodsFrom 610 Human T cell Leukemia Virus-infected patients of the Human T cell Leukemia Virus outpatient clinic from Institute of Infectious Diseases “Emilio Ribas” in São Paulo, 273 agreed to participate: 72 had HAM/TSP (excluded from this analysis) and 201 were asymptomatic, a classification performed during a regular neurological appointment. We selected the subgroup made up only by the 201 asymptomatic subjects to avoid bias by the clinical status as a confounder effect, who had laboratory results of Human T cell Leukemia Virus type 1 proviral load and T-cell proliferation assay in our database. They were further grouped according to their serological status in four categories: 121 Human T cell Leukemia Virus type 1 asymptomatic mono-infected carriers; 32 Human T cell Leukemia Virus type 1/Hepatitis C Virus, 29 Human T cell Leukemia Virus type 1/Human Immunodeficiency Virus type 1, and 19 Human T cell Leukemia Virus type 1/Human Immunodeficiency Virus type 1/Hepatitis C Virus co-infected patients. Clinical data were obtained from medical records and interviews. DNA Human T cell Leukemia Virus type 1 proviral load (PVL) and T-cell proliferation (LPA) assay were performed for all samples.
ResultsFrom a total of 273 subjects with Human T cell Leukemia Virus type 1, 80 presented co-infections: 29 had Human Immunodeficiency Virus type 1, 32 had Hepatitis C Virus, and 19 had Human Immunodeficiency Virus type 1 and Hepatitis C Virus. Comparing the groups based on their serological status, independently of being asymptomatic carriers, we observed a significant increase of PVL (p<0.001) and LPA (p=0.001). However, when groups were stratified according to their clinical and serological status, there was no significant increase in Human T cell Leukemia Virus type 1 PVL and LPA.
ConclusionNo significant increase of basal T-cell proliferation among Human T cell Leukemia Virus type 1 co-infected was observed. This interaction may be implicated in liver damage, worsening the prognosis of co-infected patients or, on the contrary, inducing a higher spontaneous clearance of Hepatitis C Virus infection in Human T cell Leukemia Virus type 1 co-infected patients.
Human T-cell lymphotropic virus type 1 (HTLV-1) is a retrovirus etiologically linked to adult T-cell leukemia/lymphoma,1,2 HTLV-1-associated myelopathy/tropical spastic paraparesis (HAM/TSP), and other inflammatory diseases.3 This virus is endemic in Japan, Caribbean Basin, and some countries in Latin America,4,5 with 5–10 million people infected worldwide.6,7 In Brazil, the highest prevalence of HTLV-1 is found in the North and Northeast, particularly in the cities of Belem, São Luiz, and Salvador.8
In areas endemic for retroviruses, the higher probability of occurrence of co-infections (HIV and HTLV-1, for example), with hepatitis C virus (HCV) may change some characteristics of the disease, such as an altered response to treatment,9 and especially in the pathogenesis of liver disease.9 Indeed, cell mediated immunity involved in the development and progression of liver disease associated with the interaction between HCV and HTLV-1 may contribute to changes in the natural history of the disease caused by these viruses, such as the development of hepatocellular carcinoma in co-infected subjects.10
In previous findings, HAM/TSP progression was associated with T-cell activation in the spinal cord, leading to an inflammatory process and demyelinization.11 A possible cause for higher immune activation could be the presence of higher DNA proviral loads (PVL) among HAM/TSP patients.12 Alternatively, those findings could be due to duration of disease, since we studied a broad range of TSP/HAM cases.13 In case of HTLV-1/HIV-1co-infection, down-regulation of T-cell proliferation, usually present with HIV infection, may not occur, a finding that could be related to the lower survival rate of such patients.14 Based on the consequences of co-infection on HAM/TSP development, we examined the possibility of an association among asymptomatic HTLV-1-co-infected subjects, increase of HTLV-1 DNA proviral load, and T-cell proliferation in a large cohort of HTLV-1-infected subjects in Sao Paulo city, Brazil.
Material and methodsThe HTLV outpatient clinic from Institute of Infectious Diseases “Emilio Ribas” (IIER) has been following a cohort of 610 HTLV-1-infected patients for 19 years, starting in July 1997. For the purpose of this study, we recruited a total of 201 HTLV-1-infected subjects who were older than 18 years and remained in active follow-up from June 2011 to May 2012, and were clinically asymptomatic. The Ethical Review Board of the IIER approved the protocol (Number 13/2011), and a signed informed consent was obtained from all participants prior to study inclusion.
All 201 volunteers were asymptomatic according to neurological evaluation and were selected if laboratory results of HTLV-1 proviral load and T-cell proliferation were available and retrievable in the patient's record. Eligible patients were classified according to their serological status in four categories: 121 HTLV-1 asymptomatic monoinfected carriers; 32 HTLV-1/HCV, 29 HTLV-1/HIV-1, and 19 HTLV-1/HIV-1/HCV co-infected patients.
Blood samples were collected in acid-citrate-dextrose solution, and PBMC were separated by Ficoll density gradient centrifugation (Pharmacia, Uppsala, Sweden). Cells were washed with saline solution; cell number was adjusted to 2×106cells and then stored at −80°C. DNA was extracted using a commercial kit (Illustra Tissue and Cells GenomicPrep Mini Spin kit, Easton Turnpike, Fairfield, CA) according to manufacturer's instructions. After this procedure the DNA was stored at −80°C for later analysis.
Quantification of HTLV-1 proviral loadThe HTLV-1 proviral load was quantified by real-time PCR using primers and probes targeting the pol gene: SK110 (5′-CCCTACAATCCAACCAGCTCAG-3′), and SK111 (5′-GTGGTGAAGCTGCCATCGGGTTTT-3′). The internal HTLV-1 TaqMan probe (5′-FAMCTTTACTGACAAACCCGACCTACCCATGGATAMRA-3′) was selected using the Oligo (version 4, National Biosciences, Plymouth, MI, USA). For quantification of the human albumin gene, the primers Alb-S (5′-GCTGTCATCTCTTGTGGGCTGT-3′) and Alb-AS (5′-AAACTCATGGGAGCTGCTGGTT-3′) and albumin TaqMan probe (5′-FAMCCTGTCATGCCCACACAAATCTCTCCTAMRA-3′) were used as described previously.15,16 Based on the median of asymptomatic individuals, 200copies/104 PBMCs of PVL was the value used as a cut off to discriminate from HAM/TSP subjects.
T-cell proliferation (LPA) assay using peripheral blood mononuclear cell cultures (PBMC)T-cell proliferation assay was performed as described in detail elsewhere.17 Briefly, 10mL of peripheral heparinized blood was collected from every patient and control, and PBMCs were isolated using Ficoll-Hypaque (Pharmacia, New Jersey, USA) gradient, washed two times in sterile saline and resuspended in RPMI 1640 (Difco, NY, USA). PBMCs from patients and controls, 2×106cells/mL in RPMI with 10% fetal calf serum were incubated at 37°C and 5% CO2 for three days with PHA and OKT3, and six days with PWM and CMA in triplicate in 96-well plates (Costar, Cambridge, MA). Cells were pulsed with tritiated thymidine (0.5μCi/mL, Amersham Int., England) 18h before harvesting in a semi-automatic cell harvester (Flow Laboratories, United Kingdom) and counted in a β-counter (Beckman, USA). The mean counts per minute (CPM) of the triplicate samples were calculated and the results expressed as the difference between the cpm of stimulated and non-stimulated cultures. The stimulation index was measured by the ratio between spontaneous/stimulated results.17
Statistical analysisStudent's t-test was used for parametric continuous variable, and the chi-square test for proportions. Possible differences in patient characteristics or laboratory values among the groups were evaluated with Mann–Whitney's test and Kruskal–Wallis test. Logistic regression analysis was performed to identify independent variables associated with HTLV-1 co-infection.
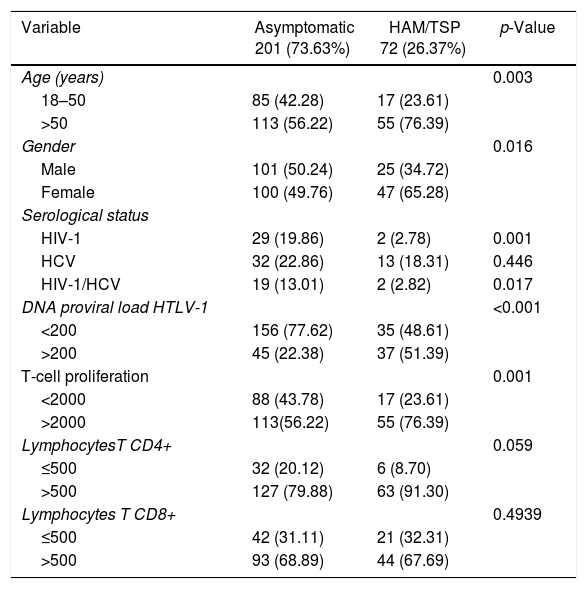
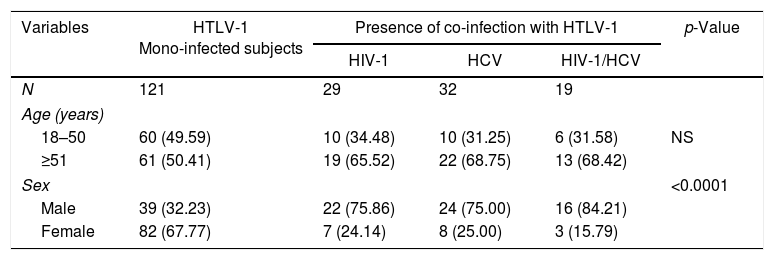
ResultsStudy subjects characteristicsThe characteristics of the participants according to disease status are presented in Table 1. Presence of HIV-1 co-infection (p=0.001) and HIV-1/HCV (p=0.017) was more frequent in the asymptomatic group. The asymptomatic group consisted of 201 subjects infected with HTLV-1 with 80 having co-infections (66.12%): 29 HIV-1 (36.25%), 32 HCV (40.0%) and 19 HIV-1 and HCV (23.75%) co-infected patients. There were more males than females (p<0.001) [Table 2]. There was no age difference between co-infected subjects monoinfected asymptomatic subjects.
Characteristics of 273 HTLV-1-infected subjects.
| Variable | Asymptomatic 201 (73.63%) | HAM/TSP 72 (26.37%) | p-Value |
|---|---|---|---|
| Age (years) | 0.003 | ||
| 18–50 | 85 (42.28) | 17 (23.61) | |
| >50 | 113 (56.22) | 55 (76.39) | |
| Gender | 0.016 | ||
| Male | 101 (50.24) | 25 (34.72) | |
| Female | 100 (49.76) | 47 (65.28) | |
| Serological status | |||
| HIV-1 | 29 (19.86) | 2 (2.78) | 0.001 |
| HCV | 32 (22.86) | 13 (18.31) | 0.446 |
| HIV-1/HCV | 19 (13.01) | 2 (2.82) | 0.017 |
| DNA proviral load HTLV-1 | <0.001 | ||
| <200 | 156 (77.62) | 35 (48.61) | |
| >200 | 45 (22.38) | 37 (51.39) | |
| T-cell proliferation | 0.001 | ||
| <2000 | 88 (43.78) | 17 (23.61) | |
| >2000 | 113(56.22) | 55 (76.39) | |
| LymphocytesT CD4+ | 0.059 | ||
| ≤500 | 32 (20.12) | 6 (8.70) | |
| >500 | 127 (79.88) | 63 (91.30) | |
| Lymphocytes T CD8+ | 0.4939 | ||
| ≤500 | 42 (31.11) | 21 (32.31) | |
| >500 | 93 (68.89) | 44 (67.69) |
PVL, absolute HTLV-1 proviral load; LPA, T-cell proliferation assay.
Demographic characteristics of 201 asymptomatic HTLV-1-mono and co-infected subjects.
| Variables | HTLV-1 Mono-infected subjects | Presence of co-infection with HTLV-1 | p-Value | ||
|---|---|---|---|---|---|
| HIV-1 | HCV | HIV-1/HCV | |||
| N | 121 | 29 | 32 | 19 | |
| Age (years) | |||||
| 18–50 | 60 (49.59) | 10 (34.48) | 10 (31.25) | 6 (31.58) | NS |
| ≥51 | 61 (50.41) | 19 (65.52) | 22 (68.75) | 13 (68.42) | |
| Sex | <0.0001 | ||||
| Male | 39 (32.23) | 22 (75.86) | 24 (75.00) | 16 (84.21) | |
| Female | 82 (67.77) | 7 (24.14) | 8 (25.00) | 3 (15.79) | |
HTLV-1 affects the immunological response and exerts an influence in T cell proliferation and T cell counts, turning the host vulnerable to the development of HAM/TSP.14 Hence, the HTLV-1 DNA proviral load has been implicated as an important factor in the development of HAM/TSP.18 Asymptomatic patients also show an increase in those factors, albeit mild, without a corresponding change in their clinical status.19 We sought to understand how the spontaneous T-cell proliferation, HTLV-1 DNA proviral load, T CD8+ and T CD4+ cell counts were assessed in asymptomatic co-infected subjects.
The PVL, expressed as the copy number of HTLV-1 proviral DNA per 104 PBMC, was significantly higher in HAM/TSP patients than in asymptomatic subjects (p<0.001), in contrast to lymphocyte T-cell proliferation (LPA) assays, since HAM/TSP patients’ lymphocytes proliferated significantly more than asymptomatic patients, as shown by their respective CPM (p=0.001).
There are some limitations to this study, since we have collected secondary data regarding T CD4+ (for 42 asymptomatic patients and for three HAM/TSP carriers) and TCD8+ (for 66 asymptomatic patients and for seven HAM/TSP subjects) from the clinical charts (Tables 1 and 3).
Proviral loads, lymphoproliferation and T lymphocytes cell counts among 201 mono and co-infected HTLV-1-infected subjects.
| Variables | HTLV-1 Mono-infected subjects | Presence of co-infection with HTLV-1 | ||
|---|---|---|---|---|
| HIV-1 | HCV | HIV-1/HCV | ||
| N | 121 | 29 | 32 | 19 |
| DNA proviral load HTLV-1 | ||||
| <200 | 95 (78.51) | 22 (75.86) | 24 (75.00) | 15 (78.94) |
| >200 | 26 (21.49) | 7 (24.14) | 8 (25.00) | 4 (21.05) |
| T-cell proliferation | ||||
| <1000 | 54 (44.63) | 12 (41.38) | 13 (40.62) | 9 (47.37) |
| >1000 | 67 (55.37) | 17 (58.62) | 19 (59.38) | 10 (52.63) |
| Lymphocytes T CD4+ count | ||||
| ≤500 | – | 12 (48.00) | 10 (38.47) | 10 (58.82) |
| >500 | 91 (100) | 13 (52.00) | 16 (61.53) | 7 (41.18) |
| Lymphocytes T CD8+ count | ||||
| ≤500 | 39 (42.86) | 1 (6.67) | 2 (10.53) | – |
| >500 | 52 (57.14) | 14 (93.33) | 17 (89.47) | 10 (100) |
PVL, HTLV-1 DNA proviral load; LPA, basal lymphocyte proliferation assay.
No differences between monoinfected and co-infected subjects as to T-cell proliferation and HTLV-1 DNA proviral loads were observed (Table 3). On the same Table, it is apparent that T CD8+ and T CD4+ cell counts of mono and co-infected patients were different, with the former presenting higher counts of both types of cells, but a high number of missing values for both variables precluded a statistical analysis to be done.
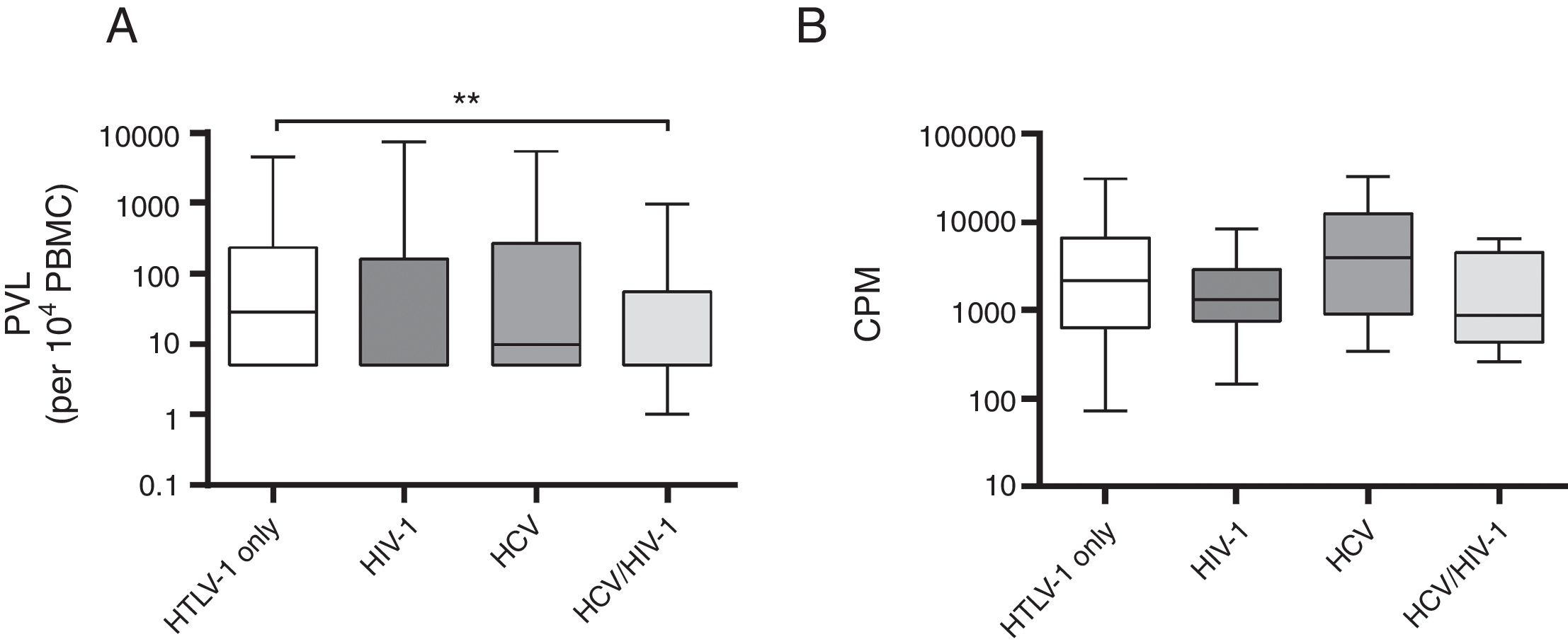
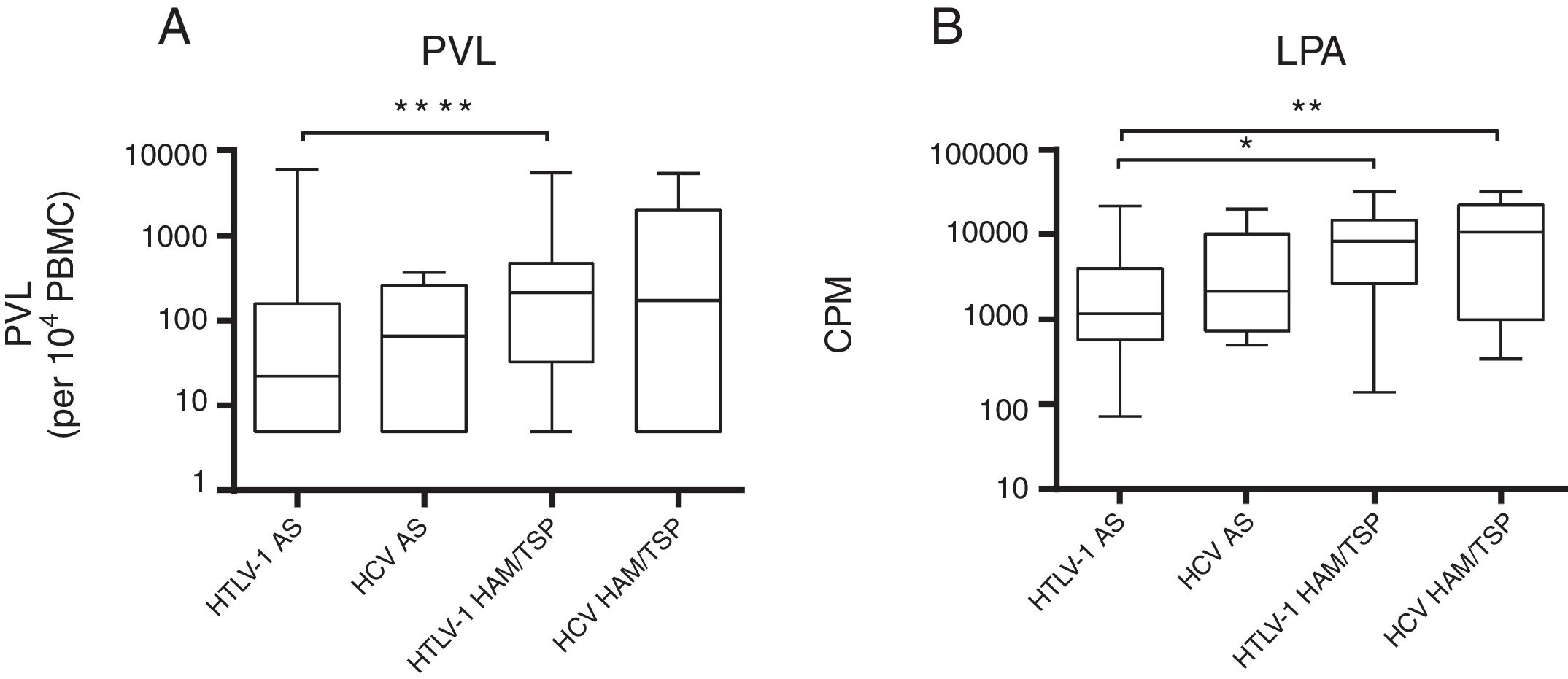
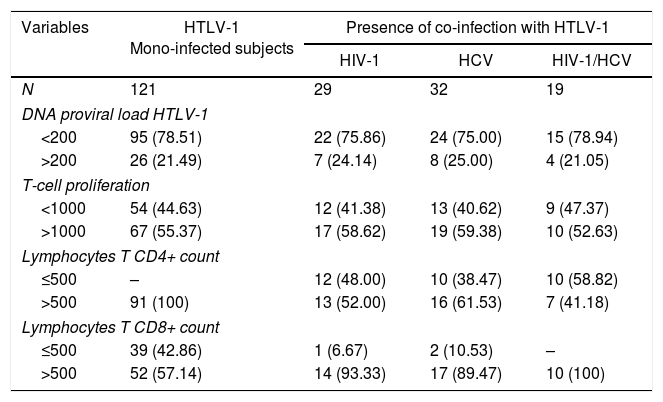
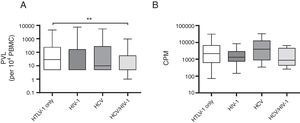
A comparison of mono and co-infected groups of patients, independently of being asymptomatic carriers, showed a significant increase in PVL (p=0.006) and LPA(p=0.01) (Fig. 1). However, when patients were stratified based on their clinical and serological status, no significant increase of HTLV-1 PVL and T-cell proliferation compared to HTLV-1 asymptomatic monoinfected subjects with HAM/TSP could be observed (Fig. 2A–D). Fig. 2A shows a significant difference between HTLV-1 asymptomatic monoinfected subjects compared to HTLV-1/HIV-1 asymptomatic carriers.
HTLV-1 Proviral load and lymphoproliferation among co-infected patients. (A) PVL, HTLV-1 proviral load; B, basal T-cell proliferation; (B) CPM, counts per minute; this figure describes all HTLV-1 monoinfected subjects compared to those with co-infection, ** The PVL had a significant association when compared to HTLV-1-monoinfected group and HCV/HIV-1/HTLV-1 co-infected subjects (A). There was no difference in the level of spontaneous LPA among monoinfected or viral co-infected groups (B).
HTLV-1 proviral load and lymphoproliferation among HCV co-infected asymptomatic subjects and HAM/TSP patients. This figure depicts the subjects when stratified based on their clinical co-infection status. A**** shows an association on PVL among asymptomatic vs HAM/TSP. We observed a significant increase in HTLV-1 PVL in HTLV-1 monoinfected HAM/TSP subjects compared to HTLV-1 monoinfected asymptomatic subjects. The higher spontaneous LPA for HTLV-1. B* LPA was associated among asymptomatic vs HAM/TSP, and we can observed in B** that LPA was associated when compared asymptomatic HTLV-1 vs HCV with HAM/TSP subjects. (A) PVL, HTLV-1 proviral load; B, basal T-cell proliferation; (B) CPM, counts per minute.
Previous reports showed contradictory results regarding HTLV-1-HCV co-infection.20–22 If HTLV-1 could worsen the prognosis of HCV disease, as postulated by some authors, it would have been possible to see those poorer outcomes in a large cohort. In reality, we observed an increase in the number of co-infected subjects in our cohort over the years, making clear that co-infections have no impact on the clinical outcome of HTLV-1-infected patients. Neither T-cell proliferation nor DNA HTLV-1 proviral load were increased among HCV/HTLV-1 subjects compared to patients with HTLV-1/HIV-1 co-infection or HTLV-1/HIV-1/HCV. It seems that, even with HIV infection under control, some proteins of this virus cause a down-regulation of T-cell proliferation. On the last years, few new cases of HAM/TSP have been reported among HIV-1/HTLV-1co-infected subjects, despite the occurrence of 10 new cases on the first years of introduction of HAART.22,23
It should be pointed out the higher occurrence of co-infected cases in males. One could infer that males are more exposed to HCV and HIV infections, as intravenous drug use was more common in males in the 1990s.24 These co-infections could affect immunological parameters of HTLV-1-infected patients.
A common finding in HTLV/HIV co-infection is an increased CD4 T-cell count without any additional immunological benefit to the patient,19,25–28 with the possibility of causing an undue delay in the start of antiretroviral treatment in co-infected patients.29 It is possible that earlier diagnosis, associated with better control of HIV infection with newer antiretroviral regimens may, have contributed to reduce the occurrence of HAM/TSP among HIV/HTLV co-infected patients. It has been noted that the CD8 T cells count was increased in all co-infected patients, both with HIV and HCV. This upregulation may be due to the presence of an increased viral load or to immune dysfunction caused by other, non-identified factors.30
Nevertheless, during the acute phase of HCV infection, a potent T-cell response would be an important factor for viral clearance.24,31 However, in the chronic phase the activation of CTLs leads to progressive liver damage. Thus, anti-HCV response could be impaired in HTLV-1-infected patients, the lysis of HCV-infected hepatocytes would be minor, causing a delay in the decision to perform liver biopsy and/or initiating anti-HCV treatment.9 The higher proliferative capacity could have decreased the potential for liver damage. In fact, HCV/HTLV-1 co-infected patients may have less immune mediated liver injury, but other factors associated with HTLV-1 can influence disease progression.32,33 Furthermore, HTLV-1/HCV co-infected patients showed less hepatic injury, and similar data shown in the Miyazaki Cohort Study indicated a possible negative interaction between HTLV-1 and HCV with respect to abnormal levels of ALT, with the prevalence of elevated ALT levels being lower in co-infected subjects than in subjects with HCV alone.34 In Bahia, one study showed that HIV/HTLV-1 and HIV/HCV co-infections could worsen the clinical related outcomes, but virologic and immunologic outcomes were similar. Liver function tests were more impaired in patients with more severe immunosuppression.35 This observation is probably due to an ineffective antiviral therapy for both diseases in the past.
As for HTLV/HIV co-infection, clinical stage at diagnosis should also be carefully considered in the interpretation of clinical studies involving HTLV/HIV/HCV co-infections, since a higher CD4 count due to HTLV-1 infection may lead to bias in the stratification of patients. Moreover, chronic hepatitis C has a prolonged course and the clinical follow-up may not be long enough to allow for definitive conclusions. Current studies have reported higher spontaneous clearance of HCV infection in patients with triple infection (HTLV/HIV/HCV) than in those with HIV/HCV or HCV only.24 In fact, HTLV-1 may modulate the immune response in HTLV/HIV co-infected patients by increasing the production of gamma-interferon and other pro-inflammatory cytokines related to Th1 type response. This mechanism could bring about an enhancement of the immune response against HCV, providing conditions for increased spontaneous clearance of HCV, which depends strongly on the endogenous production of interferons.
In conclusion, we observed a non significant increase of basal T-cell proliferation among HTLV-1 co-infected patients. However, HIV-1 co-infection may induce down-regulation of T-cell proliferation capacity. This interaction may be implicated in liver damage, worsening the prognosis of co-infected patients or, on the contrary, a higher spontaneous clearance of HCV infection in HTLV-1 co-infected patients. It is possible that a decreased T-cell proliferation can be a feasible strategy to avoid HAM/TSP development or to obtain an improvement of its symptoms, as seen with the use of corticosteroids. Further studies including more patients and longer observation periods are needed to clarify this matter.
Authors’ contributionsTA performed the statistical analysis and wrote the main text; TM and MPMB performed the lab assays; AP, MH,JS, APO select the volunteers and discussed the main text; LAM and PJN revised the statistical analysis and discussion of text; JC conceived and read the final version of this manuscript.
Conflicts of interestThe authors declare no conflicts of interest.
To all participants who contributed to this study. Support: CNPq: 234058/2014-5; FAPESP: 2012/23397-0; FAPESP: 2014/22827-7.