Infective Endocarditis (IE) is a complex, life-threatening disease. The aim of the present study was to evaluate the impact of the Endocarditis-Team on management of IE. This observational study conducted at a university hospital (2015‒22), included adult patients with IE. The study period was divided in two periods: before (pre-Endocarditis-Team; pre-ET) and after the establishment of the Endocarditis-Team (post-Endocarditis-Team; post-ET) on January 2018. Among 505 IE episodes (187 in pre-Endocarditis-Team, 318 in post-ET period), 18F-Fluorodeoxyglucose Positron Emission Tomography/Computed Tomography was more commonly used in post-ET period (14 % vs. 28 %; p < 0.001). Overall, thirty-day and one-year mortality were 14 % and 27 %, respectively; no difference was observed between the two periods. In post-ET period, the administration of 4-weeks, rather than 6-weeks, of intravenous antimicrobial treatment was higher than in the post-ET period (15 % vs. 45 %; p < 0.001). Indication for surgery was present in 115 (61 %) patients in pre-ET and in 153 (48 %) in the post-ET period. In post-ET period, among patients with indication, valve surgery was more frequently performed (66 % vs. 78 %; p = 0.038). Such difference was due to a higher acceptance of operative indication by the cardiac surgeon (69 % vs. 94 %; p = 0.013). The observed increase in number of patients benefiting from cardiac surgery in the post-ET period led to a decrease of subsequent embolic events, since among patients with operative indication (n = 268), new embolic events after the establishment of the indication were more common in the pre-ET period compared to post-ET (23 % vs. 12 %; p = 0.033). After the implementation of the multidisciplinary Endocarditis-Team we observed several improvements in the general management of IE patients.
Infective Endocarditis (IE) can present with a wide range of symptoms and signs diagnosis can be challenging, as patients often present with nonspecific symptoms.1,2 Diagnosis is based on a combination of clinical symptoms/signs, microbiologic tests, including mainly blood cultures, and imaging studies. Several attempts to establish clinical criteria to diagnose IE were previously undertaken. Since their introduction in 1994, the Duke criteria and their subsequent revisions were the mainstay of diagnosis.1,3,4 However, those criteria were established for research purposes and their performance in the clinical setting remained suboptimal, especially among patients with prosthetic valve IE, or among patients with negative blood cultures due to prior antimicrobial treatment.
IE is a rare and complex disease associated with significant morbidity and mortality.1,2 Prompt identification of IE and its complications is essential for improving prognosis, since rapid establishment of appropriate antimicrobial treatment and prompt interventions such as valve surgery or Cardiac Implantable Electronic Device (CIED) removal when indicated, were associated with better outcome.1,5-7 Valve surgery is required in 40 %‒50 % of IE patients; the principal indications being acute heart failure due to acute valvular failure, uncontrolled infection and prevention of embolic events.1 The timing of surgery is critical and should be individualized based on the patient's status and the severity of the infection, with emergent surgery being recommended in patients with refractory pulmonary oedema or cardiogenic shock.1
Based on the complexity of diagnosis and management of IE patients, the 2015 European Society of Cardiology (ESC) guidelines recommended a multidisciplinary approach for the optimal management of such patients.1 The same recommendation remained also in the revised guidelines of 2023.8 The implementation of Endocarditis-Team was shown to increase the rate of surgical intervention and reduce mortality,1,9,10 but these results were not universally found.11-14
The aim of our study was to assess the impact of an Endocarditis Heart-Team approach on the diagnosis and management of IE by performing a before-and-after analysis.
Materials and methodsStudy designThe study was conducted at a university hospital, a 1100-bed primary and tertiary care hospital from January 2015 to June 2022 (2015–17: retrospective cohort, 36-months; 2018 onwards: prospective cohort, 54-months). The study was approved by the ethics committee of the Canton of Vaud (CER-VD 2017 02137).
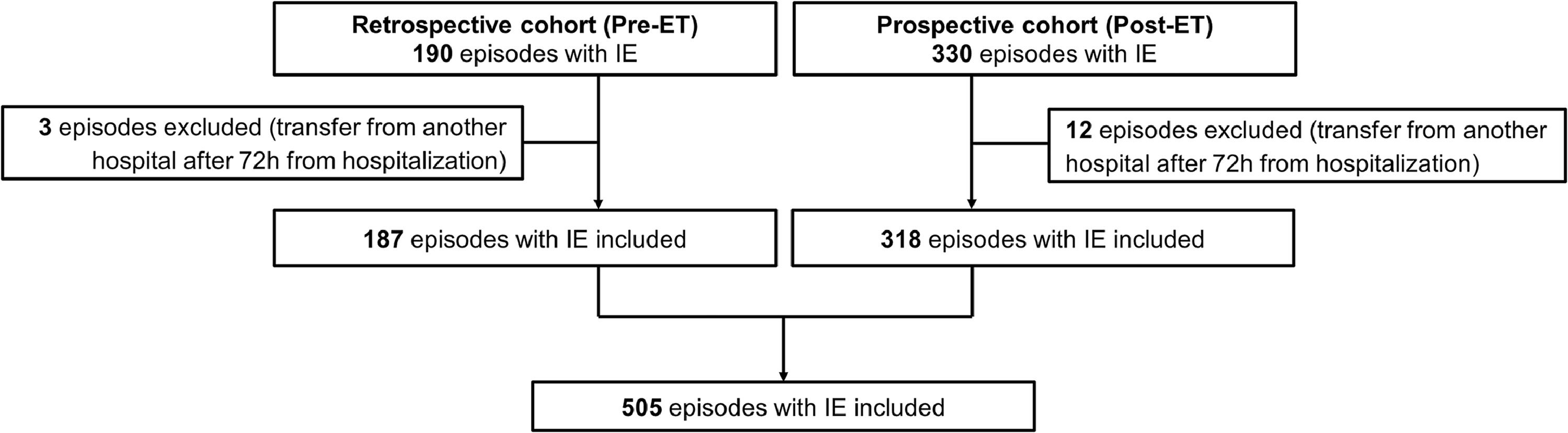
PatientsInclusion criteria were adult patients (≥ 18-years-old) and diagnosis of IE. Additional inclusion criterion for the prospective cohort was the written consent and for the retrospective cohort the absence of refusal of the use of their data. Patients that were transferred from another hospital after 72 h from hospitalization were excluded. A subsequent episode was excluded if it occurred within one-year from the initial one. All patients are followed for at least 1-year from IE diagnosis.
Data regarding demographics (age, sex), comorbidities, cardiac predisposing factors,13 CIEDs, microbiologic etiology, systemic symptoms, fever, acute heart failure, sepsis or septic shock, heart murmur, immunological phenomena,13 cardiac and non-cardiac imaging studies, site of cardiac involvement and type of lesion, cardiac surgery (timing, indication), embolic events (type, timing) and antimicrobial treatment were retrieved from patients’ electronic health records.
Management of IEAn Endocarditis-Team was established on January 2018, including infectious diseases specialists, cardiologists, and cardiac surgeons, which reviewed all patients with suspected IE suspicion during weekly meetings. Additionally, microbiologists, radiologists and specialists in nuclear medicine participated when indicated.
According to internal guidelines (before and after Endocarditis-Team establishment), an infectious diseases consultation with a thorough physical examination was performed on a mandatory basis for all patients with suspected IE. Thoraco-abdominal and cerebral imaging studies were performed in all symptomatic patients.15,16 Their realization in asymptomatic patients was left at the discretion of the treating physician and infectious diseases consultant.
DefinitionsThe study period was divided in two periods; the one before (pre-ET; from 2015 to 2017) and the other after the implementation of Endocarditis-Team (post-ET; from 2018 to 2022). In both periods, the diagnosis of IE was made on day 60 according to the 2015 ESC modified Duke criteria.13 Indications for valve surgery were also based on the aforementioned guidelines.13 The date of establishment was defined as the day on which an episode fulfilled any of the criteria outlined in the guidelines.13
EndpointsThe primary endpoint was 30-day (early) mortality. Secondary endpoints were one-year (late) mortality, realization of cardiac and non-cardiac imaging studies, realization of valve surgery when indicated, new embolic events after the establishment of operative indication and adherence to guidelines for the choice and duration of antimicrobial treatment.
AnalysisSPSS version 26.0 (SPSS, Chicago, IL, USA) software was used for data analysis. Categorical variables were analyzed using the Chi-Square or Fisher's exact test and continuous variables with Mann-Whitney U test. Based on the 2015 ESC guidelines, a duration of 4 to 6 weeks of IV antimicrobial treatment is indicated for native valve IE.13 We evaluated the duration of IV antimicrobial treatment in patients who did not require treatment for >4-weeks. For this analysis, we excluded patients with prosthetic valve IE, CIED-IE only, enterococcal IE treated with amoxicillin-ceftriaxone combination. non-cardiac infectious complications requiring IV antimicrobial treatment exceeding 4-weeks (such as cerebral or epidural abscesses), and those who died before completing 4-weeks of treatment. All statistic tests were 2-tailed and p < 0.05 was considered statistically significant.
ResultsAmong 520 IE episodes, 505 were included (15 episode were excluded since patients were transferred from another hospital after 72 h from hospitalization); 187 in the pre-ET (5.2 IE episodes per month) and the remaining 318 in post-ET period (5.9 per month) (Fig. 1).
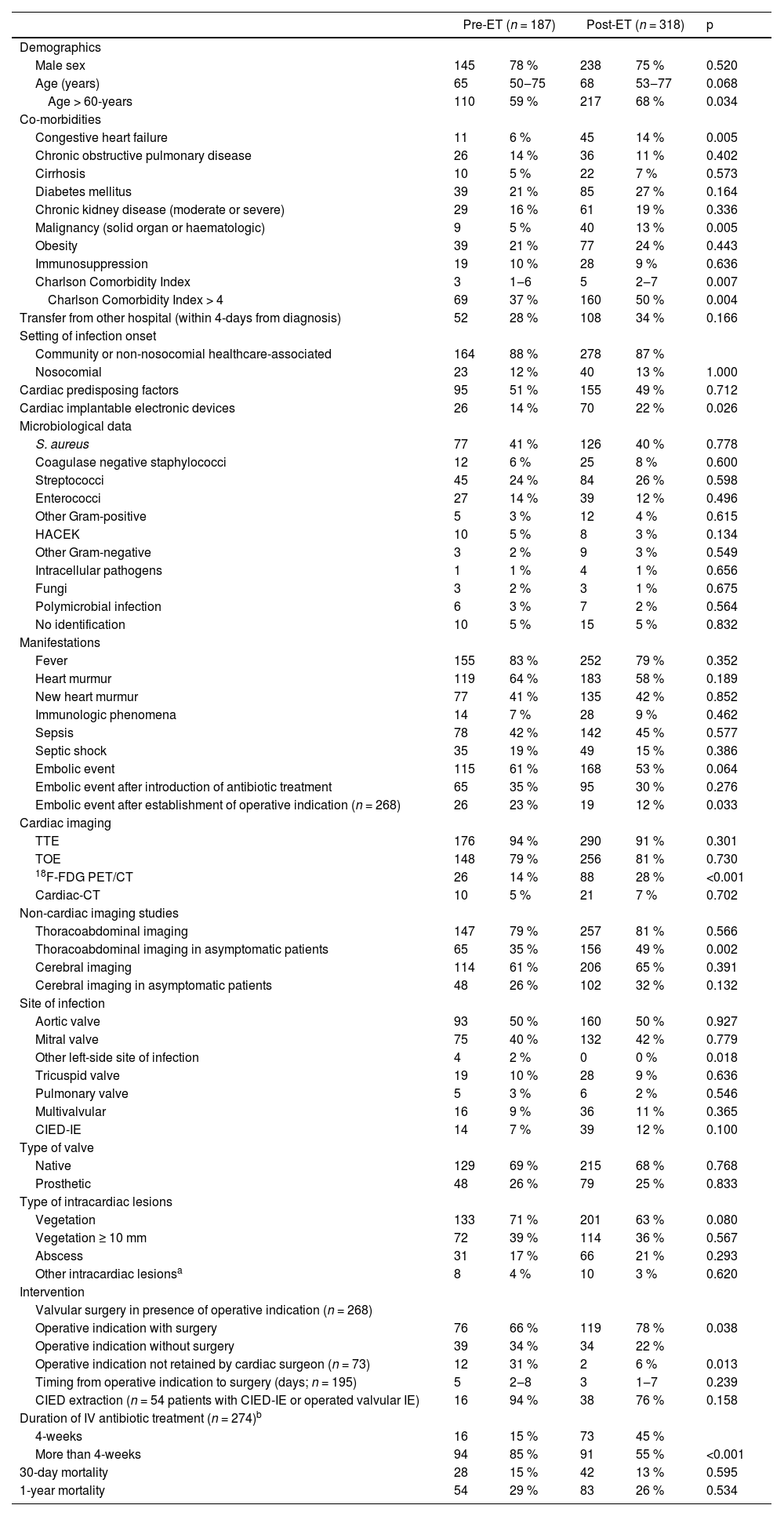
The comparison of IE patients in pre-ET and post-ET patients is shown in Table 1. Patients in post-ET were older and had higher Charlson Comorbidity Index compared to pre-ET. No difference on microbiological aetiology, manifestations, site of intracardiac infection or type of intracardiac lesions was observed between the two periods.
Characteristics of IE patients in pre-ET and post-ET periods.
| Pre-ET (n = 187) | Post-ET (n = 318) | p | |||
|---|---|---|---|---|---|
| Demographics | |||||
| Male sex | 145 | 78 % | 238 | 75 % | 0.520 |
| Age (years) | 65 | 50‒75 | 68 | 53‒77 | 0.068 |
| Age > 60-years | 110 | 59 % | 217 | 68 % | 0.034 |
| Co-morbidities | |||||
| Congestive heart failure | 11 | 6 % | 45 | 14 % | 0.005 |
| Chronic obstructive pulmonary disease | 26 | 14 % | 36 | 11 % | 0.402 |
| Cirrhosis | 10 | 5 % | 22 | 7 % | 0.573 |
| Diabetes mellitus | 39 | 21 % | 85 | 27 % | 0.164 |
| Chronic kidney disease (moderate or severe) | 29 | 16 % | 61 | 19 % | 0.336 |
| Malignancy (solid organ or haematologic) | 9 | 5 % | 40 | 13 % | 0.005 |
| Obesity | 39 | 21 % | 77 | 24 % | 0.443 |
| Immunosuppression | 19 | 10 % | 28 | 9 % | 0.636 |
| Charlson Comorbidity Index | 3 | 1‒6 | 5 | 2‒7 | 0.007 |
| Charlson Comorbidity Index > 4 | 69 | 37 % | 160 | 50 % | 0.004 |
| Transfer from other hospital (within 4-days from diagnosis) | 52 | 28 % | 108 | 34 % | 0.166 |
| Setting of infection onset | |||||
| Community or non-nosocomial healthcare-associated | 164 | 88 % | 278 | 87 % | |
| Nosocomial | 23 | 12 % | 40 | 13 % | 1.000 |
| Cardiac predisposing factors | 95 | 51 % | 155 | 49 % | 0.712 |
| Cardiac implantable electronic devices | 26 | 14 % | 70 | 22 % | 0.026 |
| Microbiological data | |||||
| S. aureus | 77 | 41 % | 126 | 40 % | 0.778 |
| Coagulase negative staphylococci | 12 | 6 % | 25 | 8 % | 0.600 |
| Streptococci | 45 | 24 % | 84 | 26 % | 0.598 |
| Enterococci | 27 | 14 % | 39 | 12 % | 0.496 |
| Other Gram-positive | 5 | 3 % | 12 | 4 % | 0.615 |
| HACEK | 10 | 5 % | 8 | 3 % | 0.134 |
| Other Gram-negative | 3 | 2 % | 9 | 3 % | 0.549 |
| Intracellular pathogens | 1 | 1 % | 4 | 1 % | 0.656 |
| Fungi | 3 | 2 % | 3 | 1 % | 0.675 |
| Polymicrobial infection | 6 | 3 % | 7 | 2 % | 0.564 |
| No identification | 10 | 5 % | 15 | 5 % | 0.832 |
| Manifestations | |||||
| Fever | 155 | 83 % | 252 | 79 % | 0.352 |
| Heart murmur | 119 | 64 % | 183 | 58 % | 0.189 |
| New heart murmur | 77 | 41 % | 135 | 42 % | 0.852 |
| Immunologic phenomena | 14 | 7 % | 28 | 9 % | 0.462 |
| Sepsis | 78 | 42 % | 142 | 45 % | 0.577 |
| Septic shock | 35 | 19 % | 49 | 15 % | 0.386 |
| Embolic event | 115 | 61 % | 168 | 53 % | 0.064 |
| Embolic event after introduction of antibiotic treatment | 65 | 35 % | 95 | 30 % | 0.276 |
| Embolic event after establishment of operative indication (n = 268) | 26 | 23 % | 19 | 12 % | 0.033 |
| Cardiac imaging | |||||
| TTE | 176 | 94 % | 290 | 91 % | 0.301 |
| TOE | 148 | 79 % | 256 | 81 % | 0.730 |
| 18F-FDG PET/CT | 26 | 14 % | 88 | 28 % | <0.001 |
| Cardiac-CT | 10 | 5 % | 21 | 7 % | 0.702 |
| Non-cardiac imaging studies | |||||
| Thoracoabdominal imaging | 147 | 79 % | 257 | 81 % | 0.566 |
| Thoracoabdominal imaging in asymptomatic patients | 65 | 35 % | 156 | 49 % | 0.002 |
| Cerebral imaging | 114 | 61 % | 206 | 65 % | 0.391 |
| Cerebral imaging in asymptomatic patients | 48 | 26 % | 102 | 32 % | 0.132 |
| Site of infection | |||||
| Aortic valve | 93 | 50 % | 160 | 50 % | 0.927 |
| Mitral valve | 75 | 40 % | 132 | 42 % | 0.779 |
| Other left-side site of infection | 4 | 2 % | 0 | 0 % | 0.018 |
| Tricuspid valve | 19 | 10 % | 28 | 9 % | 0.636 |
| Pulmonary valve | 5 | 3 % | 6 | 2 % | 0.546 |
| Multivalvular | 16 | 9 % | 36 | 11 % | 0.365 |
| CIED-IE | 14 | 7 % | 39 | 12 % | 0.100 |
| Type of valve | |||||
| Native | 129 | 69 % | 215 | 68 % | 0.768 |
| Prosthetic | 48 | 26 % | 79 | 25 % | 0.833 |
| Type of intracardiac lesions | |||||
| Vegetation | 133 | 71 % | 201 | 63 % | 0.080 |
| Vegetation ≥ 10 mm | 72 | 39 % | 114 | 36 % | 0.567 |
| Abscess | 31 | 17 % | 66 | 21 % | 0.293 |
| Other intracardiac lesionsa | 8 | 4 % | 10 | 3 % | 0.620 |
| Intervention | |||||
| Valvular surgery in presence of operative indication (n = 268) | |||||
| Operative indication with surgery | 76 | 66 % | 119 | 78 % | 0.038 |
| Operative indication without surgery | 39 | 34 % | 34 | 22 % | |
| Operative indication not retained by cardiac surgeon (n = 73) | 12 | 31 % | 2 | 6 % | 0.013 |
| Timing from operative indication to surgery (days; n = 195) | 5 | 2‒8 | 3 | 1‒7 | 0.239 |
| CIED extraction (n = 54 patients with CIED-IE or operated valvular IE) | 16 | 94 % | 38 | 76 % | 0.158 |
| Duration of IV antibiotic treatment (n = 274)b | |||||
| 4-weeks | 16 | 15 % | 73 | 45 % | |
| More than 4-weeks | 94 | 85 % | 91 | 55 % | <0.001 |
| 30-day mortality | 28 | 15 % | 42 | 13 % | 0.595 |
| 1-year mortality | 54 | 29 % | 83 | 26 % | 0.534 |
Data are depicted as number/percentage or median/Q1‒Q3.
18F-FDG PET/CT 18F-Fluorodeoxyglucose Positron Emission Tomography/Computed Tomography; CIED, Cardiac Implantable Electronic devices; ET, Endocarditis Team; HACEK, Haemophilus spp, Aggregatibacter spp, Cardiobacterium hominis, Eikenella corrodens, Kingella kingae; IE, Infective Endocarditis; TTE, Transthoracic Echocardiography; TOE, Transesophageal Echocardiography.
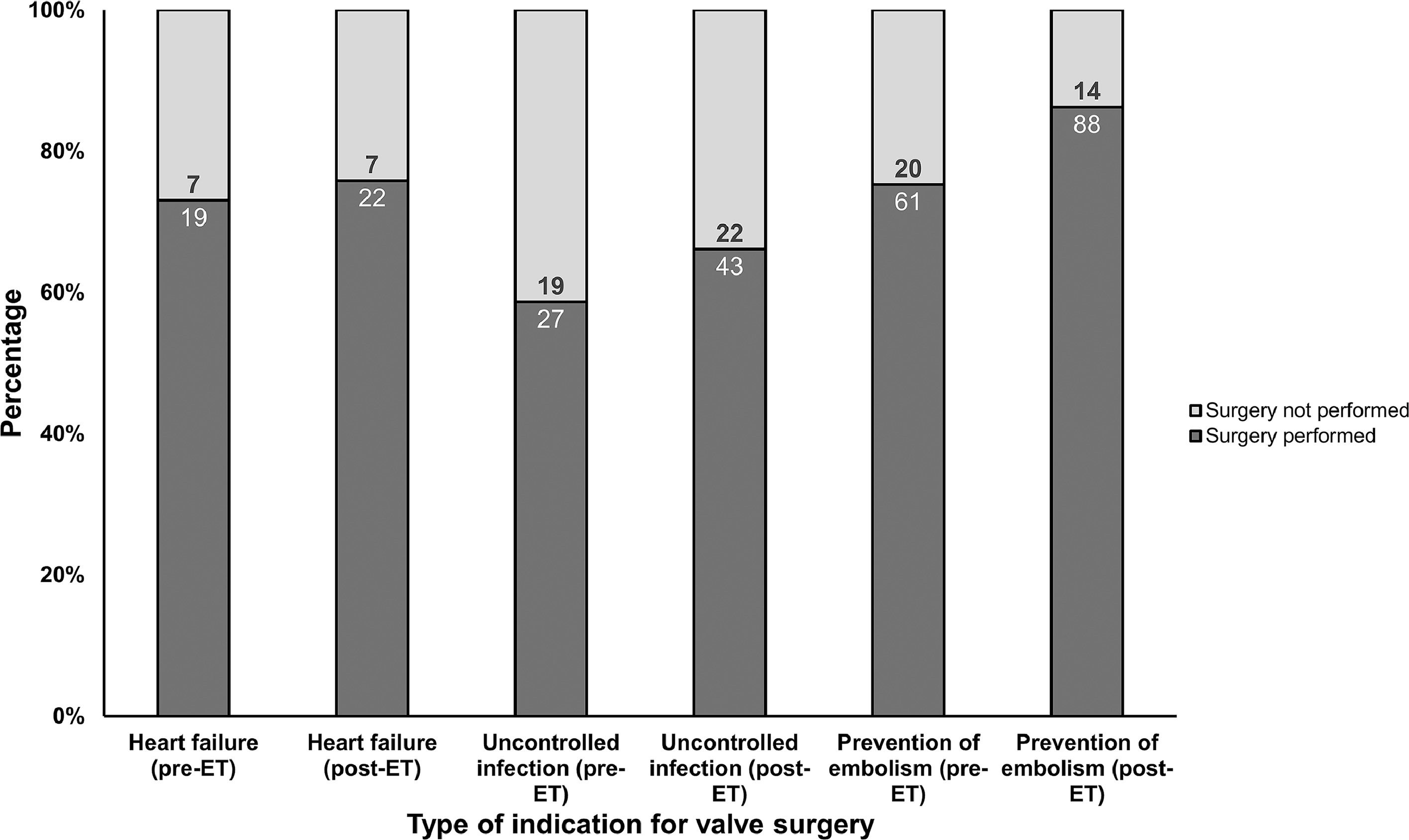
Thirty-day and one-year mortality were 14 % and 27 %, respectively. No difference on early and late mortality was observed between pre-ET and post-ET periods. In post-ET period, the administration of 4-weeks of IV antimicrobial treatment was higher than in the pre-ET (15 % vs. 45 %; p < 0.001). Indication for surgery was present in 115 (61 %) patients in pre-ET and in 153 (48 %) post-ET. In post-ET, valve surgery was more frequently performed (66 % vs. 78 %; p = 0.038) among patients with indication (Fig. 2). Such difference was due to a higher acceptance of operative indication by the cardiac surgeon in the post-ET period (69 % vs. 94 %; p = 0.013). Among patients with operative indication (n = 268), new embolic events after the establishment of the indication were more common in the pre-ET period compared to post-ET period (23 % vs. 12 %; p = 0.033).
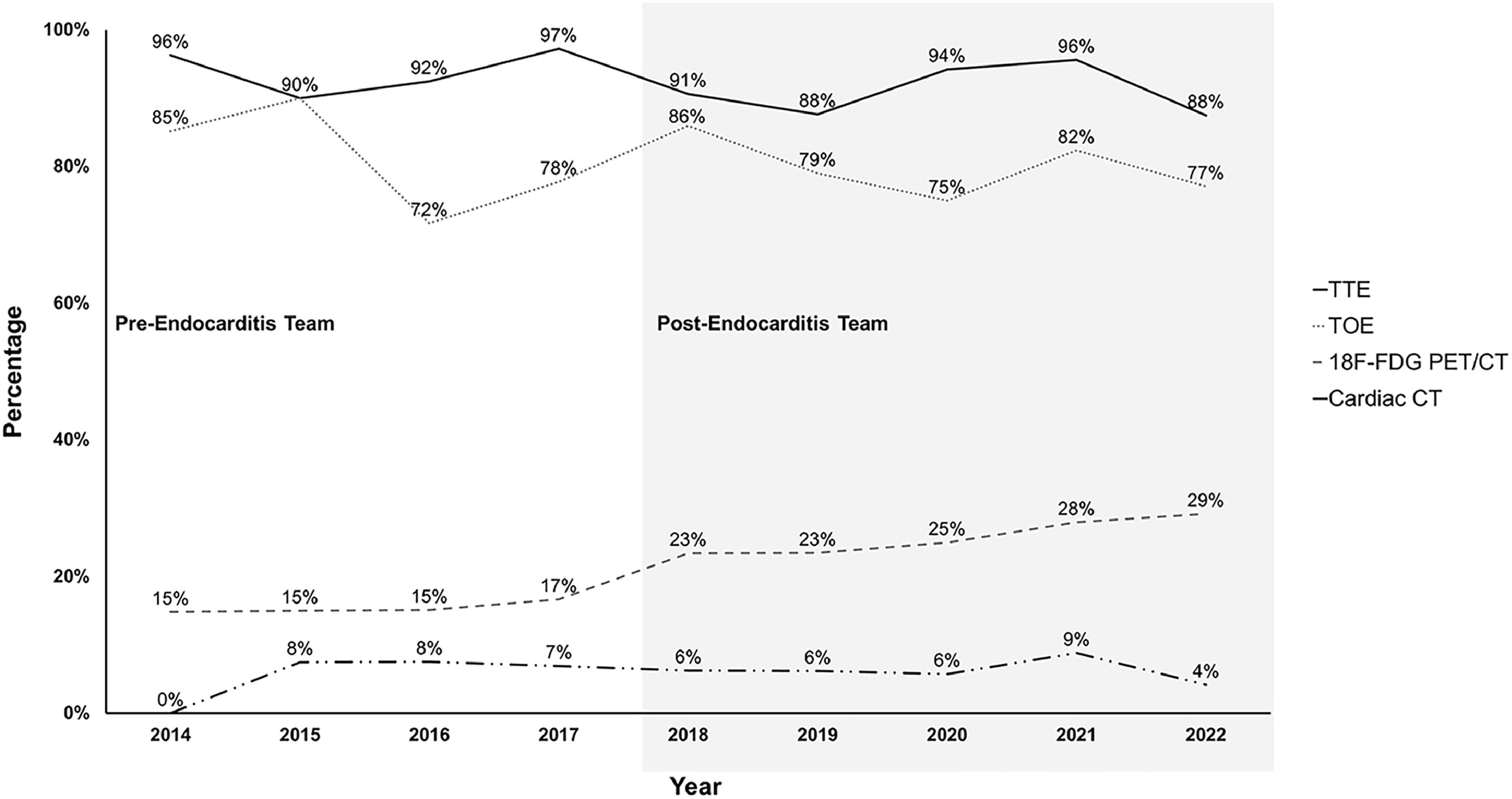
No difference was observed between the two periods on rate of performance of transthoracic or transesophageal echocardiograms, cardiac CT and thoracoabdominal or cerebral imaging studies (Fig. 3). In post-ET period, 18F-Fluorodeoxyglucose Positron Emission Tomography/Computed Tomography (18F-FDG-PET/CT) was more commonly used (14 % vs. 28 %; p < 0.001).
DiscussionThe present study demonstrated improvements in the management of IE patients (diagnosis, antibiotic treatment, surgery) after the introduction of an Endocarditis-Team.
Despite the improvement in diagnosis, medical and surgical management in the post-ET period, we did not find an improvement on survival. Our study showed comparable mortality rates to previous ones.11-14,17 Only three of the aforementioned studies found a decrease in mortality in post-ET period;91718 in one after applying a propensity score the impact on mortality dissipated.17 In a meta-analysis of studies on management of IE, the implementation of multidisciplinary teams was associated with decreased short-term mortality.19 The absence of impact of the Endocarditis-Team on mortality in the present study, can be explained by the fact that in the pre-ET period, all IE patients were followed by an infectious diseases consultant who acted as the intermediary for other consultants such the cardiologist and cardiac surgeon, thus a more informal type of “Endocarditis-Team” existed before the creation of the official Endocarditis-Team. In most of previous studies, no information on the management of IE patients in the pre-ET period was mentioned.9,11,12,14,17,18,20-22 In our institution, infectious diseases consultation among patients with Staphylococcus aureus bacteraemia, of which 14 % had IE, was associated with better outcome, highlighting the importance of such intervention.23
The main finding of the present study was an increase in the number of valve surgery performed among patients with an operative indication.24 The observed increase in valve surgery in the post-ET period was due to higher acceptance of operative indication by the team of cardiac surgery during the weekly meetings. The indications that were dismissed by cardiac surgeons in the pre-ET related to prevention of embolism. Such indications have minimal effect on mortality, but can impact morbidity by decreasing further embolic events.25 Previous studies showed no significant increase on surgical management,9,12,13,17,18,20-22 but some exhibited that valve surgery in the post-ET period was performed earlier than in the pre-ET.12,17 In the present study, even though valve surgery was performed earlier in the post-ET period (3-days from operative indication establishment) compared to pre-ET period (5-days), this did not achieve statistical significance (p = 0.239). Another explanation for the lack of impact of the Endocarditis Team on mortality in the present study might be that even in the pre-ET period, patients were operated on earlier than reported in prior studies (median of 5-days vs. 6‒14).6,13,24
The increase in valve surgery in the post-ET period could explain the decrease in further embolic events observed in the present study.13 No study to date evaluated the role of Endocarditis-Team in outcomes other than mortality or length of stay. By investigating a wider range of outcomes beyond mortality, we could gain a more comprehensive understanding of the effectiveness of the multidisciplinary approach in managing infective endocarditis.
Another finding was the observed shortening on the antibiotic treatment duration. The 2015 ESC guidelines propose that among patients with native valve IE due to staphylococci and enterococci a duration of 4 to 6 weeks, and for streptococci 4-weeks.1 We noted that in patients with native valve IE not necessitating an extension of IV treatment beyond 4-weeks, there was an increase in the proportion of individuals receiving a 4-week course of IV antibiotic therapy, from 15 % in the pre-ET to 45 % in the post-ET (p < 0.001). The duration of antibiotic treatment was seldomly reported in previous studies,12,20 with conflicting results; one study showed no difference on antibiotic treatment duration,20 while another showed a significant decrease of antibiotic treatment duration in the post-ET,12 and in a third all patients in pre- and post-ET periods received appropriate antimicrobial duration.24
The establishment of the Endocarditis-Team did not impact the rates of transthoracic and transoesophageal echocardiograms, which were high in both periods. In two studies, an increase in transoesophageal echocardiograms was found in post-ET.9,11 Following evidence regarding an improvement in the diagnosis of prosthetic valve IE by 18F-FDG PET/CT and the recommendation of the 2015 ESC guidelines,1,26,27 an increase in the realization of aforementioned imaging study was observed in the post-ET period. While between 2014 and 2017, the utilization of 18F-FDG PET/CT ranged from 15 % to 17 %, this rate increased to 23 % in 2018. Such an increase in 18F-FDG PET/CT in the post-ET was also observed in a previous study.9 The 2015 ESC guidelines also recommend considering non-cardiac imaging studies (thoracoabdominal or cerebral) for the detection of embolic events in patients with a high clinical suspicion but for whom IE diagnosis is not yet proven, even though they might not offer a diagnostic advantage.1,15 In the present study, Endocarditis-Team did not influence the rate of such imaging studies.
Another role of the Endocarditis Team extended beyond the management of IE patients to include research activities. The team adjudicated whether patients with suspected IE, had or not IE based on microbiological, clinical, imaging, surgical, and pathological findings presented at weekly meetings. This process served as a reference standard for evaluating different versions of the Duke criteria and various prediction scores used to diagnose IE in patients with bacteremia caused by typical microorganisms.28-30
Our study has several limitations. First, this is a single-center, observational study, with a moderate number of patients, even though in the present study the study size was significantly higher than most previous studies.9,11,12,14,17,18,20-22 The difference of type of inclusion could offer a bias, since after 2018 patients were included in a prospective manner. In the prospective cohort, 88 % of eligible patients provided informed consent and were consequently included in the study. Similarly, in the retrospective cohort, 91 % of eligible patients were included, with only 9 % having not sign the general informed consent. This high inclusion rate suggests a robust representation of patients in both cohorts, minimizing potential biases related to patient selection. Second, since all patients in the pre-ET were followed by an infectious disease's specialist, the real impact of an Endocarditis-Team approach may be underestimated. Therefore, the present results must be generalized with caution. Last, patients in the pre-ET period were included retrospectively; in order to minimize the bias, patients with IE were identified by three different approaches: 1) ICD-10 coding in the discharge letter, 2) Cardiac surgery and CIED-removal, and 3) Bacteraemia by typical IE pathogens. Another limitation was that some of the differences observed between the two time periods could be explained due to advances on IE diagnosis of IE; however, concerning 18F-FDG PET/CT, there was an abrupt increase in 2018, probably attributable to the Endocarditis-Team presence.
ConclusionsThe implementation of a multidisciplinary Endocarditis-Team offered several improvements in the overall management of IE patients, which included increased utilization of advanced imaging studies, such as 18F-FDG PET/CT, a reduction in the duration of IV antimicrobial treatment and expansion of the number of patients benefiting from cardiac surgery. Although these changes did not have a discernible impact on early or late mortality, they did lead to a significant decrease in subsequent embolic events attributed to a higher number of patients undergoing valve surgery. To comprehensively evaluate the impact of a multidisciplinary Endocarditis-Team, it is imperative to conduct further prospective, multicenter studies that explore a wide range of outcomes beyond mortality.
Data availabilityThe data that support the findings of this study are available from the corresponding author upon reasonable request.
Authors’ contributionsMPO and PM conceived the idea. NF, VZ, BG, NI, PT, MK and MPO collected the patients' data. PM supervised the project. MPO performed the analysis and interpreted the results. NF and VZ wrote the manuscript. All authors contributed to manuscript revision and read and approved the submitted version.
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.