The World Health Organization influenza forecast now includes an influenza B strain from each of the influenza B lineages (B/Yamagata and B/Victoria) for inclusion in seasonal influenza vaccines. Traditional trivalent influenza vaccines include an influenza B strain from one lineage, but because two influenza B lineages frequently co-circulate, the effectiveness of trivalent vaccines may be reduced in seasons of influenza B vaccine-mismatch. Thus, quadrivalent vaccines may potentially reduce the burden of influenza compared with trivalent vaccines.
In this Phase III, open-label study, we assessed the immunogenicity and safety of Southern Hemisphere inactivated quadrivalent influenza vaccine (Fluarix™ Tetra) in Brazilian adults (NCT02369341). The primary objective was to assess hemagglutination-inhibition antibody responses against each vaccine strain 21 days after vaccination in adults (aged ≥18–60 years) and older adults (aged >60 years). Solicited adverse events for four days post-vaccination, and unsolicited adverse events and serious adverse events for 21 days post-vaccination were also assessed.
A total of 63 adults and 57 older adults received one dose of inactivated quadrivalent influenza vaccine at the beginning of the 2015 Southern Hemisphere influenza season. After vaccination, in adults and older adults, the hemagglutination-inhibition titers fulfilled the European licensure criteria for immunogenicity. In adults, the seroprotection rates with HI titer ≥1:40 were 100% (A/H1N1), 98.4% (A/H3N2), 100% (B/Yamagata), and 100% (B/Victoria); in older adults were 94.7% (A/H1N1), 96.5% (A/H3N2), 100% (B/Yamagata), and 100% (B/Victoria). Pain was the most common solicited local adverse events in adults (27/62) and in older adults (13/57), and the most common solicited general adverse events in adults was myalgia (9/62), and in older adults were myalgia and arthralgia (both 2/57). Unsolicited adverse events were reported by 11/63 adults and 10/57 older adults.
The study showed that inactivated quadrivalent influenza vaccine was immunogenic and well-tolerated in Brazilian adults and older adults.
Traditional trivalent influenza vaccines (inactivated trivalent influenza vaccines [IIV3s] and live attenuated influenza vaccines [LAIV3s]) include two influenza A strains (A/H1N1 and A/H3N2) and one influenza B strain that are recommended annually for the Northern and Southern Hemispheres by the World Health Organization (WHO) based on global surveillance. However, in the early 1980s, two phylogenetically-distinct influenza B lineages (B/Yamagata and B/Victoria) emerged globally in humans.1 In a report published by The Global Influenza B Study in 2015, it was estimated that based on 26 countries in both Hemispheres and inter-tropical regions between 2000 and 2013, the average rate of influenza B lineage mismatch with the seasonal vaccine was about 25%.2
Although data regarding the burden of influenza B in Latin America are limited, influenza B has circulated in Brazil during most seasons over the past decade and B lineage has been reported in some seasons.3–7 Surveillance studies between 2001 and 2013 show that one B lineage predominated in 10 seasons, and co-circulation was found in 2002, 2008, and 2013.5 During 2013, when the B/Victoria strain in the Southern Hemisphere seasonal vaccine was mismatched, the reported rate of influenza B mismatch with the vaccine was >91% in Brazil, 100% in São Paulo, and 52% in South America.3–6
In response to global reports of B lineage mismatch and the hypothesis that quadrivalent vaccine may reduce the burden of influenza disease compared with trivalent vaccine, for the first time in the 2012–2013 Northern Hemisphere influenza season, the WHO influenza forecast included an influenza B strain from each of the influenza B lineages.8,9 By the 2014–2015 influenza season, various quadrivalent influenza vaccines had been launched globally, and depending upon the region and product, were licensed for use in adults and children from 6 months of age.10–20
This Phase III, open-label study was conducted to assess the immunogenicity and safety of Southern Hemisphere inactivated quadrivalent influenza vaccine (IIV4) in adults in Brazil.
MethodsThis Phase III, open-label study assessed the immunogenicity and safety of IIV4 in adults (18–60 years) and older adults (>60 years). The study was conducted in two centers in Brazil in 2015. Eligible subjects were aged ≥18 years, were in stable health, and had not received any non-registered drug or vaccine within 30 days, or any investigational or approved influenza vaccine within six months of the first visit. All subjects provided written informed consent.
The study protocol, informed consent and other information requiring pre-approval were reviewed and approved by Comissão Nacional de Ética em Pesquisa. The study was conducted in accordance with Good Clinical Practice, the principles of the Declaration of Helsinki, and all regulatory requirements (NCT02369341).
ObjectivesThe primary objective was to assess vaccine immunogenicity based on hemagglutination-inhibition (HI) antibody responses against each vaccine strain 21 days after vaccination in adults and older adults. Immunogenicity outcome measures (defined below) were assessed according to the European Committee for Medicinal Products for Human Use (CHMP) licensure criteria for immunogenicity of influenza vaccines.21 The secondary immunogenicity objective was to assess HI antibody responses in each age strata according to previous influenza vaccine history during the preceding influenza season (2014) and according to baseline serostatus.
Reactogenicity and safety were assessed as secondary objectives and included solicited adverse events (AEs) (reactogenicity) for four days post-vaccination and unsolicited AEs, serious AEs (SAEs) and medically-attended AEs (MAEs) for 21 days post-vaccination.
VaccineThe inactivated split virion vaccine (Fluarix™ Tetra) was a thiomersal-free vaccine manufactured by GSK Vaccines in Dresden, Germany. One 0.5mL dose contained 60μg hemagglutinin antigen (HA) including each of the four vaccine strains recommended by WHO for the 2015 influenza season in the Southern Hemisphere: A/California/7/2009 (H1N1)pdm09-like strain [variant A/Christchurch/16/2010 (NIB-74xp)], A/Switzerland/9715293/2013 (H3N2)-like strain [variant A/Switzerland/9715293/2013 (NIB-88)], B/Phuket/3073/2013-like strain [B/Phuket/3073/2013] (Yamagata lineage), B/Brisbane/60/2008-like strain [B/Brisbane/60/2008] (Victoria lineage).
Pre-filled syringes contained one dose of IIV4 which was administered intramuscularly in the deltoid of the non-dominant arm.
AssessmentsImmunogenicityBlood samples were collected for immunogenicity assessments before vaccination (Day 0) and 21 days (Day 21) after vaccination. Antibody titers against the vaccine strains were measured in serum samples using HI assays which were performed at a GSK Vaccines laboratory in Quebec, Canada using standardized procedures as previously described.22
Geometric mean titers (GMTs) were obtained by computing the anti-log of the arithmetic mean of the log transformed inverse titers. Subjects with HI antibody titers ≥1:10 were considered to be seropositive. HI antibody responses were described as seroconversion rate (SCR, defined as the percentage of subjects who had pre- and post-vaccination titers of <1:10 and ≥1:40, respectively, or a pre-vaccination titer of ≥1:10 and ≥4-fold increase in post-vaccination titer), seroprotection rate (SPR, defined as the percentage of subjects with a post-vaccination titer of ≥1:40), and the mean geometric increase (MGI, defined as the geometric mean of the ratio between pre-vaccination and post-vaccination reciprocal HI titers).
Reactogenicity and safetyThe occurrence, intensity, and duration of solicited AEs were recorded by subjects on diary cards for 4-days post vaccination and included injection site AEs (pain, redness, and swelling) and general AEs (arthralgia, fatigue, gastrointestinal symptoms, headache, generalized myalgia, shivering, and fever). Injection site reactions were considered to be related to vaccination and investigators provided causality assessments for solicited general AEs. Solicited events were graded for severity using a standard three grade scale: Grade 1, ‘no effect on normal activities’ (‘>20 to 50mm’ for injection site redness and swelling); Grade 2, ‘some interference with normal everyday activities’ (‘>50 to 100mm’ for injection site redness and swelling); and Grade 3, ‘prevents normal everyday activities’ (‘>100mm’ for injection site redness and swelling). Fever was graded as: Grade 1 ≥38.0 to ≤38.5°C, Grade 2 >38.5 to ≤39.0°C, and Grade 3 >39.0°C. Unsolicited AEs including medically-attended events (MAEs) and serious adverse events (SAEs) were assessed for 21 days post-vaccination. Unsolicited AEs were coded using the Medical Dictionary for Regulatory Activities Preferred Terms. Investigators provided causality assessments for all unsolicited events.
StatisticsAt least 50 evaluable subjects in each age strata (18–60 years and >60 years) were to be assessed for immunogenicity based on regulatory requirements for the annual registration of influenza vaccines (CPMP/BWP/214/96).21 Target enrolment was 60 subjects, in each age strata.
The vaccine fulfills the European CHMP licensure criteria for immunogenicity if at least one criteria was met: point estimate for SPR >70%, SCR >40%, and MGI >2.5 (adults aged 18–60 years), or SPR >60%, SCR >30%, and MGI >2.0 (older adults >60 years).21 All immunogenicity outcomes were tabulated with 95% confidence intervals (CIs). The immunogenicity analyses were performed on the per-protocol immunogenicity cohort including all eligible subjects without protocol deviation who had serological data available at Day 0 and Day 21.
Solicited and unsolicited AEs were tabulated with 95% CIs. Reactogenicity and safety analyses were performed on all vaccinated subjects (total vaccinated cohort).
ResultsThe first subject was enrolled on 29 April 2015 and all subjects had completed the study by 3 June 2015.
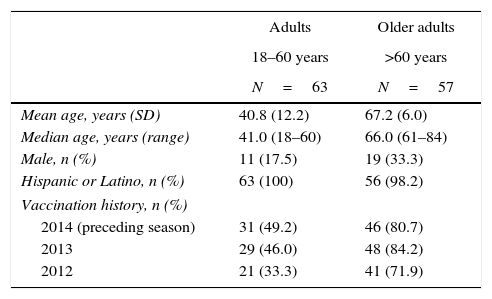
A total of 121 subjects were enrolled, and 120 were vaccinated (total vaccinated cohort), including 63 adults and 57 older adults. The per-protocol immunogenicity cohort included of a total of 119 subjects (62 adults and 57 older adults) who completed the study. One subject in the adult group withdrew from the study (moved from the study area). The mean (range) age in the adult group was 40.8 years (18–60 years), and in the older adult group was 67.2 years (61–84 years) (Table 1). Thirty-one of 63 adults and 46 of 57 older adults had received influenza vaccination in the preceding season.
Baseline characteristics in the total vaccinated cohort.
| Adults | Older adults | |
|---|---|---|
| 18–60 years | >60 years | |
| N=63 | N=57 | |
| Mean age, years (SD) | 40.8 (12.2) | 67.2 (6.0) |
| Median age, years (range) | 41.0 (18–60) | 66.0 (61–84) |
| Male, n (%) | 11 (17.5) | 19 (33.3) |
| Hispanic or Latino, n (%) | 63 (100) | 56 (98.2) |
| Vaccination history, n (%) | ||
| 2014 (preceding season) | 31 (49.2) | 46 (80.7) |
| 2013 | 29 (46.0) | 48 (84.2) |
| 2012 | 21 (33.3) | 41 (71.9) |
SD, standard deviation; N, number of subjects in group; n, number of subjects with characteristic.
At baseline in the adult group, 82.3% and 83.9% of subjects were seropositive for A/H1N1 and A/H3N2, respectively, and the seropositivity rate for both B/Yamagata and B/Victoria was 95.2%. At baseline in the older adult group, the seropositivity rate was 91.2% for both A/H1N1 and A/H3N2, and was 100% for both B/Yamagata and B/Victoria. At Day 21 post-vaccination, 100% of adults and older adults were seropositive for all vaccine strains.
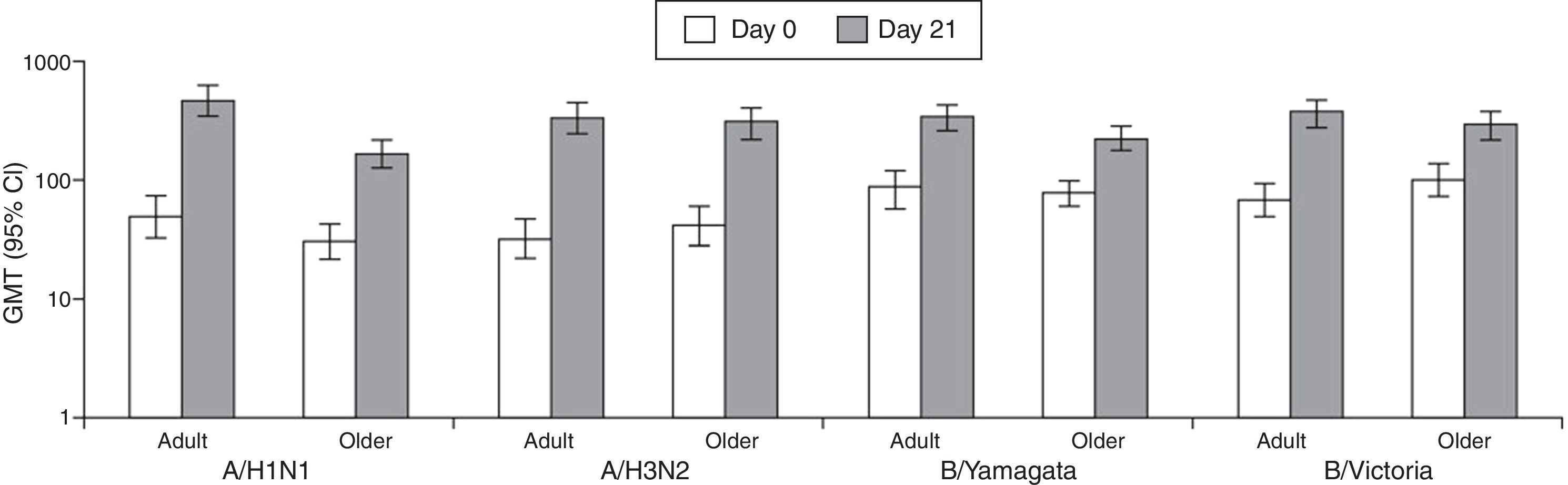
At Day 21 post-vaccination, GMTs in adults were 460.2 (A/H1N1), 338.4 (A/H3N2), 338.4 (B/Yamagata), and 365.9 (B/Victoria), and in older adults were 166.9 (A/H1N1), 306.7 (A/H3N2), 226.3 (B/Yamagata), and 288.6 (B/Victoria) (Fig. 1).
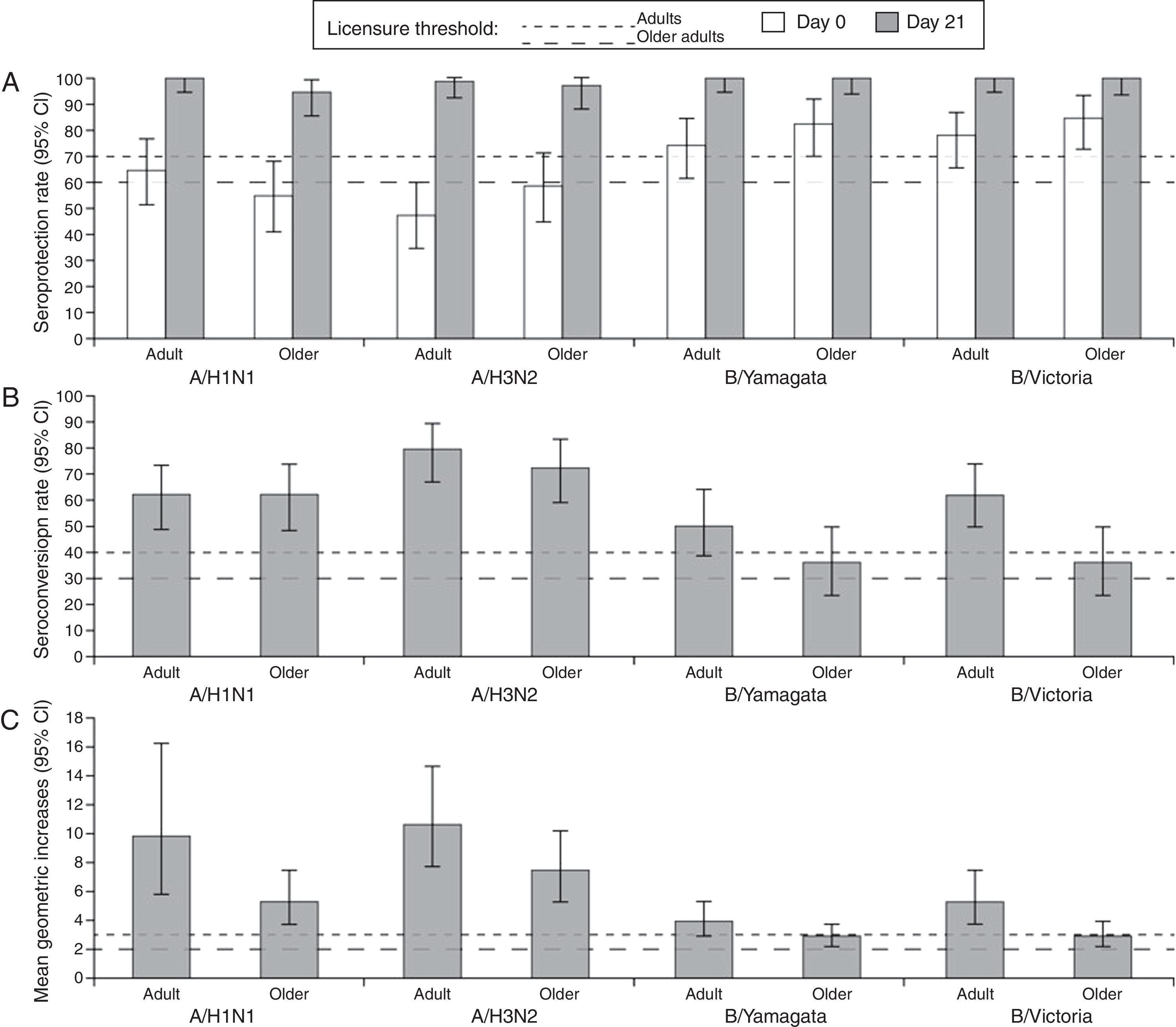
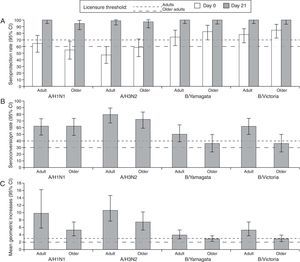
At Day 21 post-vaccination, the SCRs, SPRs, and MGIs fulfilled the European CHMP licensure criteria for immunogenicity (Fig. 2). Point estimates for SPRs in adults were 100% (A/H1N1), 98.4% (A/H3N2), 100% (B/Yamagata), and 100% (B/Victoria), and in older adults were 94.7% (A/H1N1), 96.5% (A/H3N2), 100% (B/Yamagata), and 100% (B/Victoria).
Summary of hemagglutination-inhibition seroconversion rates (A), seroprotection rates (B), and mean geometric increase (C) in the per-protocol immunogenicity cohort. CI, confidence interval; SPR, seroprotection rate; SCR, seroconversion rate; MGI, mean geometric increase; European CHMP licensure criteria for immunogenicity defined as at least one criteria to be met from: point estimate for SPR >70%, SCR >40% and MGI >2.5 (adults aged 18–60 years) or SPR >60%, SCR >30%, and MGI >2.0 (older adults >60 years).21
A summary of HI immunogenicity by vaccination history is shown in Supplement 1. In adults who had received seasonal influenza vaccine in the preceding season, the baseline seropositivity rate was 96.8–100% compared with 67.7–100% in subjects who had not received the vaccine. In the older adults who had received seasonal influenza vaccine in the preceding season, the baseline seropositivity rate was 91.3–100% compared with 81.8–100% in those who had not received the vaccine. In adults and older adults who had received seasonal influenza vaccine in the preceding season, the vaccine was immunogenic at Day 21, with SPRs of 100% and ≥93.5%, respectively. The SCRs for the B/Yamagata strain were relatively low in previously vaccinated adults and older adults (25.8% and 26.1%, respectively). In adults and older adults who had not received seasonal influenza vaccine in the preceding season, the vaccine was immunogenic at Day 21 against all vaccine strains.
In adults who were seronegative at baseline, the SCR was 90–100% and in adults who were seropositive at baseline, the SCR was 47.5–76.9%. In older adults who were seronegative at baseline, the SCR for A/H1N1 and A/H3N2 was 90.0% and 79.0%, respectively. No older adult was seronegative for influenza B strains at baseline. In older adults who were seropositive at baseline, for A/H1N1 and A/H3N2, the SCR was 59.6% and 100%, respectively, and 35.1% for each influenza B strain.
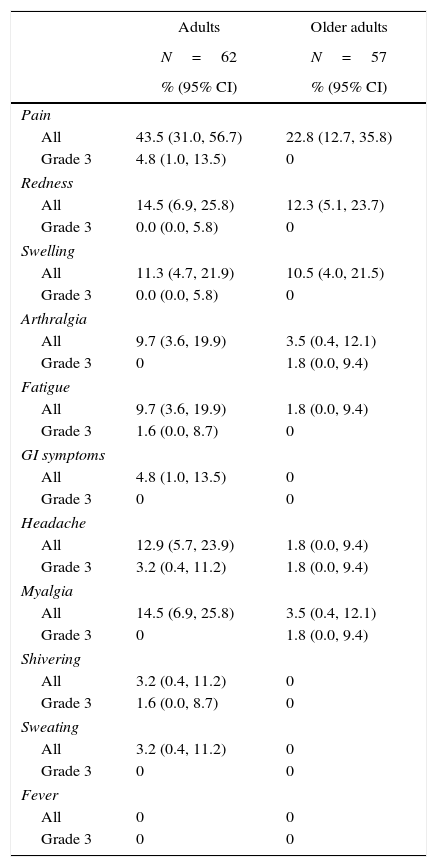
ReactogenicityFor 4-day post-vaccination, pain was the most common solicited injection site AE in adults (43.5%; 27/62) and in older adults (22.8%; 13/57). In adults, the rate of redness and swelling was 14.5% (9/62) and 11.3% (7/62), respectively, and in older adults, the rate of redness and swelling was 12.3% (7/57) and 10.5% (6/57), respectively. Grade 3 injection site AE pain was reported by 4.8% of the adult group (Table 2).
Solicited injection site adverse events and general adverse events during the 4-day post-vaccination period in the total vaccination cohort.
| Adults | Older adults | |
|---|---|---|
| N=62 | N=57 | |
| % (95% CI) | % (95% CI) | |
| Pain | ||
| All | 43.5 (31.0, 56.7) | 22.8 (12.7, 35.8) |
| Grade 3 | 4.8 (1.0, 13.5) | 0 |
| Redness | ||
| All | 14.5 (6.9, 25.8) | 12.3 (5.1, 23.7) |
| Grade 3 | 0.0 (0.0, 5.8) | 0 |
| Swelling | ||
| All | 11.3 (4.7, 21.9) | 10.5 (4.0, 21.5) |
| Grade 3 | 0.0 (0.0, 5.8) | 0 |
| Arthralgia | ||
| All | 9.7 (3.6, 19.9) | 3.5 (0.4, 12.1) |
| Grade 3 | 0 | 1.8 (0.0, 9.4) |
| Fatigue | ||
| All | 9.7 (3.6, 19.9) | 1.8 (0.0, 9.4) |
| Grade 3 | 1.6 (0.0, 8.7) | 0 |
| GI symptoms | ||
| All | 4.8 (1.0, 13.5) | 0 |
| Grade 3 | 0 | 0 |
| Headache | ||
| All | 12.9 (5.7, 23.9) | 1.8 (0.0, 9.4) |
| Grade 3 | 3.2 (0.4, 11.2) | 1.8 (0.0, 9.4) |
| Myalgia | ||
| All | 14.5 (6.9, 25.8) | 3.5 (0.4, 12.1) |
| Grade 3 | 0 | 1.8 (0.0, 9.4) |
| Shivering | ||
| All | 3.2 (0.4, 11.2) | 0 |
| Grade 3 | 1.6 (0.0, 8.7) | 0 |
| Sweating | ||
| All | 3.2 (0.4, 11.2) | 0 |
| Grade 3 | 0 | 0 |
| Fever | ||
| All | 0 | 0 |
| Grade 3 | 0 | 0 |
CI, confidence interval; N, number of subjects in group; fever defined as ≥38.0°C, Grade 3 >39.0°C; adults, aged 18–60 years; older adults, aged >60 years.
For four days post-vaccination the most common solicited general AEs in adults were myalgia (14.5%; 9/62) and headache (12.9%; 8/62), and there were two reports of Grade 3 headache and one report each of Grade 3 fatigue and shivering (Table 2). In older adults the most common general AEs were myalgia and arthralgia (both 3.5%; 2/57). Grade 3 arthralgia, headache, and myalgia were reported by one older adult (Table 2).
SafetyFor 21 days post-vaccination, in the adults, the rate of unsolicited AEs was 17.5% (11/63), including Grade 3 unsolicited AEs in four subjects which were pruritus (n=1), urinary tract infection (n=1), and sinusitis (n=2). In older adults, the rate of unsolicited AEs was 17.5% (10/57), including Grade 3 AEs in two subjects which were upper respiratory tract infection (n=1) and syncope (n=1).
There was one SAE experienced by an 84-year old subject which was syncope, reported eight days post-vaccination, which resolved by the end of the study and which was not considered by the investigator to be related to vaccination.
DiscussionThis Phase III, open-label study showed that IIV4 was immunogenic and well-tolerated in adults (18–60 years) and older adults (>60 years) in Brazil. HI antibody titers 21 days after vaccination fulfilled European CHMP licensure criteria for immunogenicity for each vaccine strain in each age group, with SPRs of >98% in adults, and >94% in older adults. In adults, the SCRs for all strains were >50%, and in older adults were >60% for influenza A and >35% for influenza B. The baseline seropositivity rate (titer ≥1:10) was high (>80%) against all strains in adults, and the entire older adult group were seropositive for the B/Yamagata and B/Victoria strains and was high with >90% for the influenza A strains. The safety profile was consistent with that observed for IIV4s from the same manufacturer in previous Phase III studies in adults and older adults.17,20
In Brazil, influenza vaccination is routinely available to adults over 60 years of age and this was reflected in our study by the rates of influenza vaccination in the preceding two seasons in older adults (80.7%) compared with adults (49.2%). However, baseline seropositivity rates were high in both age groups, and the relatively high baseline seropositivity rates we observed in adults, reflects that the vaccine strains had been circulating during the previous season. In both age groups, in subjects who had received vaccine in the preceding season, the vaccine was immunogenic with almost all subjects achieving seroprotective HI titers (≥1:40). However, among previously vaccinated subjects, the proportion achieving a four-fold increase in HI titer (SCR) was relatively low (25–80%), particularly for the B/Yamagata strain at 25.8% in adults and 26.1% in older adults.
High baseline antibody titers resulting from natural exposure or previous vaccination are reported to reduce antibody responses after seasonal influenza vaccination, such as in older populations.23–28 When vaccine immunogenicity is studied in populations that has high seropositivity pre-vaccination, it may be difficult to demonstrate an improved immune response by looking at the SCR alone. To assess the effect of baseline antibodies we could analyze SCRs according to baseline seropositivity status. In our study, we showed that in adults and older adults, SCRs were generally higher against influenza A strains in subjects who were seronegative at baseline (80–100%) compared with subjects who were seropositive (52.9–76.9%). In adults who were seronegative for influenza B strains at baseline, the SCR against both strains was 100%, compared with 47.5–59.3% in adults who were seropositive at baseline. However, all older adults were seropositive for both influenza B strains at baseline, and SCRs for B/Yamagata and B/Victoria were both relatively low at 35.1%. Although baseline antibody titers against vaccine strains were higher and immune responses were lower in older compared with younger adults, HI antibody responses against all vaccine strains fulfilled licensure criteria in both populations. These findings are consistent with observations in Phase III randomized trials of Northern Hemisphere IIV4 in adults and older adults, which showed that responses against influenza B strains were lower in subjects who had received IIV3 in the preceding three seasons than in subjects who had not.17,20,25
During the development of IIV4s, it was hypothesized that reactogenicity could be higher with the quadrivalent vaccine that contained 60μg of HA than with the IIV3s, which contained 45μg HA. Global Phase III studies showed that the reactogenicity and safety profiles of IIV4s were consistent with IIV3s, and the inclusion of an additional 15μg HA did not appear to compromise safety.17,20 In a recent report from the Vaccine Adverse Event Reporting System including reports for IIV3s and IIV4s from July 2013 to March 2015, most of the reported AEs were non-serious, and IIV3s and IIV4s had similar safety profiles.29
In our study, the solicited and unsolicited AE profile suggested that the vaccine was generally well tolerated. Solicited AEs were more frequent in adults than older adults, but Grade 3 AEs were infrequent (≤4.8% of all doses in adults and ≤1.8% of all doses in older adults). No subjects withdrew from the study for safety reasons and there was one SAE (syncope eight days post-vaccination) experienced by an 84 year old subject which resolved by the end of the study and was not considered by the investigator to be related to vaccination.
A limitation of this study was that it included adults in stable health including chronic conditions and the results may not be generalizable to ‘highest-risk’ groups such as the very frail, those with severe co-morbidities, obese people, and post-partum women. A further limitation was that, although HI antibody levels fulfilled the immunogenicity criteria used by regulatory agencies to assess the suitability of seasonal influenza vaccines, immunogenicity is a surrogate outcome for clinical protection.
Because influenza B is less prone to antigenic shift than influenza A, it is thought to be clinically less important than influenza A infections. However, influenza B epidemics are likely to be substantial since they occur in about half of influenza seasons globally, including South America.6,9,30,31 In an analysis of surveillance data in Brazil from 2000 to 2008, influenza-like illness accounted for 4.39–16.9% of all hospital consultations, and in 2008, 43.2% of confirmed influenza cases were influenza B.32 Based on the Global Influenza B Study, across seasons from 2004 to 2012 in Brazil, the average influenza A attack rate was higher (73.1%) than the rate of influenza B (26.9%), which was consistent with influenza viral circulation patterns globally.2 Moreover, the co-circulation of both B lineages and vaccine mismatch with the influenza B lineage in the recommended Southern Hemisphere IIV3 has been observed in Brazil, including the 2013 season, when the rate of B lineage mismatch with the lineage included in the vaccine was 91.4%.3–7
Modeling studies suggest that switching from trivalent to quadrivalent vaccines could reduce the number of medical visits and hospitalizations, antibiotic prescriptions written for influenza, and the economic impact of work absenteeism. In a static model developed by the US Centers for Disease Control and Prevention (CDC), the burden of influenza B was estimated across 10 seasons, and switching from trivalent to quadrivalent vaccines could have resulted in an annual reduction of 1–321 per 100,000 influenza cases, and 0.06–2.7 per 100,000 fewer influenza-related hospitalizations depending upon the epidemic intensity of influenza B during each season.8 The CDC model was adapted and used to assess the cost of switching from trivalent to quadrivalent vaccines from a Brazilian healthcare and societal perspective from 2010 to 2013.33 It was estimated that the use of quadrivalent instead of trivalent vaccine in Brazil would have reduced the number of influenza cases by 654,018, reduced the number of hospitalizations by 7536, and reduced influenza-related deaths by 1122. In 2013 when the rate of mismatch of IIV3 with the circulating influenza B strain was >90%, the model showed that quadrivalent vaccine could have avoided influenza-related costs of R$ 1 million to the health system and R$ 62 million to society.33
In summary, this Phase III, open-label study showed that Southern Hemisphere IIV4 was immunogenic and well-tolerated in adults from Brazil.
Trademarks statementsFluarix is a trademarks of the GSK group of companies.
FundingGlaxoSmithKline Biologicals SA funded this study and was involved in all stages of study conduct, including analysis of the data and funded all costs associated with the development and publication of this manuscript.
AuthorshipAll authors participated in the design or implementation or analysis, and interpretation of the study; and the development of this manuscript. All authors had full access to the data and gave final approval before submission.
Conflicts of interestJyoti Soni and Opokua Ofori-Anyinam are employed by the GSK group of companies. Varsha K Jain reports she was employed by the GSK group of companies at the time of the study, and is now employed by Bill & Melinda Gates Foundation. Ping Li reports she was employed by the GSK group of companies at the time of the study, and is now employed by Pfizer. Ping Li, Varsha K Jain and Opokua Ofori-Anyinam hold shares in the GSK group of companies. Cristiano Zerbini declares grants for research from the GSK group of companies, Lilly, Pfizer, Amgen, Merck, Sanofi, Novartis and Celltrion; and Advisory board for Lilly, Pfizer and Sanofi. Rodrigo Ribeiro dos Santos and Maria Jose Nunes declare no conflicts of interest.
The authors are indebted to the participating study volunteers, clinicians, nurses and laboratory technicians at the study sites. In particular, we thank Antonio Tarcisio Freire who provided support and care to study participants. The authors thank the teams of GSK Vaccines, in particular, Silvija Jarnjak, Els Praet, Bhakthi Pereira, Eliana de Barros, Eduardo de Gomensoro, Kanika Dey, Andreia Neves and Tom Herremans. Finally, the authors thank Annick Moon (Moon Medical Communications Ltd, UK; on behalf of GSK Vaccines) for providing medical writing services, and Bruno Dumont and Véronique Gochet (Business and Decision Life Sciences, on behalf of GSK Vaccines) for editorial assistance and manuscript coordination.