To evaluate the influence of hepatitis C virus on immunological and virological responses after highly active antiretroviral therapy initiation in human immunodeficiency virus/hepatitis C virus coinfected patients compared to monoinfected human immunodeficiency virus-infected patients.
MethodsThe study enrolled 65 human immunodeficiency virus-1-infected subjects who initiated highly active antiretroviral therapy and attended follow-up visits over 48 weeks from 2008 to 2010. They were grouped based on hepatitis C virus-RNA results. Virological and immunological responses were monitored at baseline and at the end of weeks 12, 24, 36, and 48.
ResultsThere were 35 human immunodeficiency virus monoinfected and 30 human immunodeficiency virus/hepatitis C virus coinfected patients. In the present study human immunodeficiency virus/hepatitis C virus coinfection did not seem to influence CD4 T-lymphocytes recovery. There was no difference between the curves of CD4 T-lymphocytes raise of coinfected and monoinfected groups.
ConclusionThis prospective study confirms that hepatitis C virus infection does not seem to be associated with impaired CD4 T-lymphocytes recovery after HAART.
Coinfection of hepatitis C virus (HCV) in patients with human immunodeficiency virus (HIV) has become a frequent situation, achieving a prevalence of nearly 30%, owing to common modes of transmission.1,2 In particular subgroups, as intravenous drug users and hemophiliacs, rates may rise as high as 90% and 100%, respectively.3,4
Clinical implications of HIV and HCV coinfection have been the focus of extensive research and controversial data. Several studies suggest a more rapid immune deterioration (lower levels of CD4 T-lymphocytes); higher risk for progression to acquired immunodeficiency syndrome (AIDS); increased HCV load; and a more severe liver disease.5–7
The introduction of highly active antiretroviral therapy (HAART) for the treatment of HIV-infected individuals has shown to decrease morbidity and mortality, as a result of suppression of HIV viremia, recovery of CD4 T-lymphocytes, and restoring immune function. Nonetheless, regarding the HCV coinfection, combined therapy yielded conflicting results.6 Some observational studies have found that HIV/HCV coinfected patients have an impaired CD4 T-lymphocytes recovery compared with HIV monoinfected patients after initiating HAART,5,8 whereas others have not found such an effect.6,9,10 Most studies have compared HIV/HCV populations with CD4 T-lymphocytes count over 200cells/mm3. After extensive search at Medline, the authors found no report in the literature about immune restoration in coinfected immunosupressed patients, with CD4 T-lymphocytes count near 100cells/mm3.
In this prospective study we aimed to assess the CD4 T-lymphocytes count recovery after HAART in HIV and HCV coinfected patients comparing to HIV monoinfected patients.
Individuals and methodsStudy populationThis was a prospective study conducted at a tertiary hospital in Porto Alegre, Rio Grande do Sul, in Brazil, which included patients from August 2008 through August 2010 initiating HAART and who achieved virological response in months 3 (reduction of 2-logs or higher in HIV viral load [VL]) and 6 (undetectable HIV VL). This criterion was chosen to avoid poor adherence to HAART. All patients included were previously identified as HIV infected and hepatitis B virus negative. The sample was categorized in two groups – HIV monoinfected patients and HIV/HCV coinfected patients for whom demographic characteristics, clinical and laboratory values, and medical history were recorded at enrollment (before HAART initiation) and at medical consultation every 3 months thereafter. Hepatic fibrosis was evaluated with liver biopsy, using METAVIR score.11
Laboratory testingBlood samples were collected at enrollment and every 3 months, including liver panel (alanine aminotransferases [ALT], aspartate aminotransferases [AST], albumin), HIV VL and CD4 T-lymphocytes. Serologic testing for HCV infection was performed through ELISA (Abbott Axsym System, N. Chicago, IL, EUA) and polymerase chain reaction (PCR) for HCV-RNA, through AMPLICOR qualitative test (Roche Diagnostics, Nutley, NJ, USA; detection limit: 50UI/mL).
Statistical analysisAll statistical analyses were performed using the SPSS® 10.0 statistical package. The qualitative variables were described in absolute frequency and percentage. The quantitative variables were described in means and standard deviation. For the analysis, we used the chi-squared test, Spearman correlations, and the t-test for comparison of the variables in the study. For the evaluation of analysis of variance we used Generalized Estimating Equations.
EthicsAll individuals included agreed to join the study protocol. Hospital Nossa Senhora da Conceição Ethical Committee approved the present study in 04/08/2008, number 136/08.
ResultsBaseline characteristicsFrom a total of 97 HIV-infected patients initially recruited, 65 (67%) started HAART as their first antiretroviral regimen and were included in the study. Of the patients who returned for the second consultation, there was no dropout. Baseline characteristics of the groups are summarized in Table 1. Thirty patients (46%) were HCV positive – all of them were positive for HCV-RNA, and 35 (54%) patients were HCV-RNA negative. At the end of 48 weeks of follow-up, only three (10%) patients did not reach the end point in the coinfected HIV/HCV group and three patients in the monoinfected group (8.6%). One patient (1.5%) discontinued therapy due to severe gastrointestinal symptoms, and five patients (7.5%) discontinued for unexplained reasons. Those patients had their data included in the analysis until they had virological confirmation of discontinuation of therapy.
Baseline characteristics of the study population prior to HAART introduction.
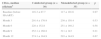
| Coinfected group (n=30) | Monoinfected group (n=35) | p | |
|---|---|---|---|
| Male, n (%) | 22 (73%) | 23 (66%) | >0.05 |
| Age (years) | 41.8±7.9 | 38.8±10.1 | 0.09 |
| Body Mass Index (BMI) | 23.6±2.1 | 24.3±3.6 | 0.68 |
| CD4+, median (cells/mm3) | 116.3±87.7 | 117±101.6 | 0.97 |
| HIV RNA (copies/mL) | 182,000 | 207,000 | 0.56 |
Intravenous drug use was the main route of HCV infection in the coinfected group, with a total of 20 patients (64.5%). The percentage of males was very similar in both groups. Comparing coinfected with monoinfected patients, the HCV infected group had higher mean age, but the difference was not statistically significant (41.8 versus 38.8 years). None of the patients included were using alcohol or drugs during the study.
Regarding HAART, in the coinfected group the regimen was two nucleoside-analog reverse-transcriptase inhibitor (NRTI) plus non-nucleoside-analog reverse-transcriptase inhibitor (NNRTI) in 21 (70%) of the patients versus 30 (85%) in the monoinfected group. Only 9 (30%) patients of the coinfected group were on two NRTI and a protease inhibitor (PI) versus 5 (15%) monoinfected patients.
Regarding coinfected group, patients were predominantly of HCV genotype 1 (n=22, 73%). No patient had diagnosis of cirrhosis. Hepatic biopsy, evaluated by METAVIR score,9 was performed in 16 of the 30 coinfected patients, and 12% had hepatic fibrosis grade 1, 50% had grade 2 and 6% had grade 3. Thirty-two percent had no fibrosis (grade 0) on hepatic biopsy.
HIV viral load responseCoinfected and monoinfected patients had similar HIV VL before initiation of HAART (182,000×207,000copies/mL, p=0.51). Because of the study design, as shown in the “Methods” section, all patients had reduction of at least of 2 logs in month 3 and undetectable HIV VL in month 6. The magnitude of decline in HIV-1 VL in both groups after HAART initiation was similar.
Immunological responseImmunological response in both groups was assessed by CD4 T-lymphocytes counts after HAART introduction and at every three months until the end of 12-month period.
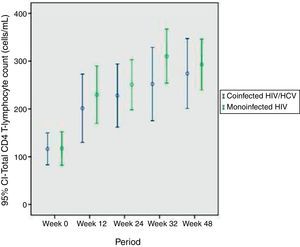
There were no statistical differences in CD4 T-lymphocytes counts between the groups during the entire study, using Bonferroni correction. Changes in the CD4 T-lymphocytes subsets are summarized in Table 2 and Fig. 1. At the beginning of HAART 13 coinfected (44%) and 20 monoinfected patients (57%) had CD4 T-lymphocytes below 100cells/mm3.
CD4 T-lymphocytes variation over 12 months of evaluation.
| CD4+, median cells/mm3 | Coinfected group (n=30) | Monoinfected group (n=35) | p |
|---|---|---|---|
| Baseline (before HAART) | 116.3±87.7 | 117±101.6 | 0.97 |
| Month 3 | 201.4±176.9 | 230±158.4 | 0.53 |
| Month 6 | 228±152.8 | 251±140.2 | 0.58 |
| Month 9 | 252.1±168.8 | 311±143 | 0.21 |
| Month 12 | 274.1±184.9 | 293±144.9 | 0.67 |
After 48 weeks of treatment with HAART, there was a significant increase in the percentage and absolute number of CD4 T-lymphocytes, in both groups, but no statistically significant differences were found.
Using Generalized Estimating Equations, the most appropriate test for evaluation of behavior of CD4 T-lymphocytes variation during the 48 weeks, there was no difference between the groups (p=0.402). CD4 T-lymphocytes curves of coinfected and monoinfected patients after HAART introduction were similar, as shown in Fig. 1.
DiscussionThe present study intended to examine the impact of HCV infection on the extent of CD4 T-lymphocytes recovery after HAART initiation in a cohort of HIV-1/HCV coinfected patients compared to HIV-1 monoinfected patients.
Our study differs from previous studies because we evaluated actually immunosuppressed patients (CD4 T-lymphocytes count around 100cells/mm3) and we assessed CD4 T-lymphocytes increase only in patients with virological response in three months (reduction of 2-logs or higher in HIV VL) and six months (undetectable HIV VL). This approach has some clear advantages once it eliminates the influence of potential differences in HAART adherence and drug potency between HCV infected and HCV uninfected patients.
There are conflicting results as to whether HCV infection does or does not have a deleterious effect on the course of HIV infection. However, most authors support a delay in CD4 T-lymphocytes recovery in HIV/HCV coinfected when compared to HIV monoinfected patients.5,8,12,13 Antonucci et al. support a direct role of HCV viremia as a potential limitation of CD4 T-lymphocytes response to HAART.8 Santín et al. suggest that HIV/HCV coinfection blunts early immune restoration during treatment with HAART, with a delay in the CD4 T-lymphocytes recovery in HIV/HCV coinfected patients.5 Potter et al. believes that CD4 cell progression is negatively affected by the presence of HCV replication. Additionally they suggest that active HCV infection affects immune restoration in coinfected patients even after years of HAART exposure.13 Many authors support the concept that after HIV-1 viral replication is kept under control, those differences in CD4 T-lymphocytes recovery disappeared, ranging from one to four years in different studies.5,12,14
On the other hand, Sulkowski et al. in a prospective cohort study of 1955 coinfected patients found no difference in the increase in CD4 T-lymphocyte counts during HAART in HCV infected compared with HCV uninfected patients. Also, no difference was detected in the risk of acquiring an AIDS-defining illness or the risk of death when comparing both groups.15 Fuping et al. evaluated 175 HIV/HCV coinfected and HIV monoinfected patients suggesting that HCV/HIV coinfection does not affect immunological and virological responses to HAART. However, HCV coinfected patients were at higher risk for drug reactions, such as rash and hepatotoxicity, to all HAART regimens,16,17 especially those treated with Nevirapine or Ritonavir.7,18,19
In the present study, we provide evidence that HIV/HCV coinfection does not seem to influence CD4 T-lymphocyte recovery in these patients compared with HIV monoinfected patients. The evaluation of analysis of variance with Generalized Estimating Equations between groups showed no difference between CD4 T-lymphocytes increment of coinfected and monoinfected groups.
Because of the massive prevalence of genotype 1 in HCV-infected patients, it was not possible to assess differences of immunological restoration according to HCV genotype. It is possible that inclusion of more patients or longer follow-up could reveal significant differences between the groups, but with the present study, we can rule out large and clinically relevant differences. Additionally absence of HCV viremia is an important limitation of our study, since it would interfere in CD4 T-lymphocytes restoration.
ConclusionTo the best of our knowledge, this is the first prospective study that evaluated immunosuppressed patients, with CD4 T-lymphocytes count around 100cells/mm3. Most studies published have included patients with higher levels of CD4 T-lymphocytes at baseline.
Finally, data from this prospective study confirm that HCV infection does not seem to be associated with impaired CD4 T-lymphocytes response to HAART.
Conflict of interestThe authors declare no conflict of interest.