Maintaining a right balance between Th17 and Treg might be critical to the immunopathogenesis of active tuberculosis (TB). This study aimed to assess whether the Th17/Treg balance is altered in active TB patients.
Methods250 study subjects (90 active TB patients, 80 latent TB subjects, and 80 healthy controls) were recruited for the study. The expression of Th17 and Treg in peripheral blood mononuclear cells (PBMCs) in the 250 subjects was investigated by flow cytometry. Plasma levels of cytokines IL-17 and IL-10, which are related to Th17 and Treg, respectively, were determined by ELISA.
ResultsThe percentages of Th17 and Treg in PBMCs from active TB patients were significantly higher than those from latent TB or control groups (Th17: 4.31±1.35% vs. 1.58±0.71% or 1.15±0.49%, p<0.05; Treg: 11.44±2.69% vs. 7.54±1.56% or 4.10±0.99%, p<0.05). The expression of IL-17 and IL-10 was significantly increased in active TB patients in comparison to that in latent TB or control groups (IL-17: 16.85±9.68 vs. 7.23±5.19 or 8.21±5.51pg/mL, p<0.05; IL-10: 28.70±11.27 vs. 20.25±8.57 or 13.94±9.00pg/mL, p<0.05).
ConclusionsOur study demonstrated an altered balance of Treg/Th17 in active TB patients, with higher percentages of Th17 and Treg in PBMCs. Further research on this imbalance may offer a new direction for TB treatment.
Tuberculosis (TB) caused by Mycobacterium tuberculosis (M. tuberculosis) is one of the world deadliest contagious diseases, with an estimated one-third of the global population being infected. According to the WHO 2014 Global tuberculosis report, there were approximately 9 million new cases and 1.5 million deaths from TB in 2013. The adaptive immune response mediated by antigen-specific CD4+ T cells is believed to be the major immunological defence mechanism against M. tuberculosis, and largely determines the clinical outcomes of M. tuberculosis infection.1 Th1 cell, a classic subset of CD4+ T cells, has been known to play an essential role in the clearance of M. tuberculosis infection.2 However, there remains an incomplete understanding of the immunological mechanisms underlying the individual variations (some individuals are protected from infection while others progress to disease). Recently, two other subtypes of CD4+ T cells, Th17 and regulatory T cell (Treg) have been shown to modulate immune response against M. tuberculosis infection.
Th17 is a CD4+ T cell subset distinct from Th1 and Th2 subsets, the biological function of which is related to the expression of cytokines such as IL-17 (also known as IL-17A), IL-17F, IL-21, and IL-22. Among these cytokines, IL-17, whose receptor is widely distributed in many target organs, including spleen, lung, and liver, appears to be the most important cytokine produced by Th17.3 IL-17 induces the expression of several pro-inflammatory factors, such as G-CSF and IL-6, and CXC chemokines, e.g. IL-8. It is known to promote granulopoiesis, neutrophil recruitment, and inflammation.4 This is suggested to be one of the mechanisms by which Th17 and Th17-derived cytokines participate in the immune response against various extracellular and intracellular bacteria, fungi, and viruses, as also in the development of autoimmune diseases.5 In TB disease, Th17 primarily exerts its protective effect through IL-17, which recruits neutrophils to promote granuloma formation,6 and increases the expression of chemokines, CXCL9, CXCL10, and CXCL11, which recruit IFN-γ producing cells to the infected site.7 On the other hand, over-expression of IL-17 leads to recruitment of large numbers of neutrophils to the infected site, which instead contributes to the immunopathological injury.8,9
Treg is another functionally and structurally distinct subset of CD4+ T cell, which expresses the specific transcription factor Foxp3. Treg plays a critical role in maintaining immune homeostasis and self-tolerance10 by suppressing an over-activated immune response through multiple mechanisms, including cell contact-dependent mechanisms, such as by expressing CTLA-4, which is important in inhibiting immune activation by competing for co-stimulatory ligands on T cells; and cell contact-independent mechanisms, such as secretion of suppressive cytokines, TGF-β and IL-10.11 During the initial phase of M. tuberculosis infection, Treg has a detrimental effect by delaying the onset of adaptive immune responses through inhibiting the activation of T cells in draining lymph nodes.12,13 However, in the late or chronic phase, although Treg limits the protective immune responses against M. tuberculosis, it also plays a beneficial role by reducing excessive inflammation which could damage host tissues, through inhibition of effector T cells and antigen-presenting cells.14,15
Th17 and Treg have opposing functions, with Th17 representing a pro-inflammatory subset, while Treg has an antagonistic effect, but their developmental pathways are reciprocally interconnected. Cytokines, TGF-β, IL-6, and IL-21 regulate the differentiation of Th17 and Treg from CD4+ T cell. In the absence of pro-inflammatory factors IL-6 and IL-21, TGF-β induced the expression of Foxp3 and appeared to promote differentiation into Treg. However, in the presence of IL-6 or IL-21, TGF-β induces the Th17-specific transcription factor RORγt expression, and promotes differentiation into Th17.16,17 Moreover, recent investigations have indicated that Th17 and Treg demonstrate plasticity. Inflammatory mediator IL-6 or components of pathogens could induce the conversion of Treg into Th17.18 Therefore, retaining the immune balance of Th17 and Treg appears to be important for host homeostasis under normal physiological conditions. Altered balance of Th17/Treg may play a critical role in the pathogenesis of various autoimmune diseases and chronic inflammatory diseases, such as TB.
Current evidence regarding the association of Th17/Treg imbalance and disease development mainly focuses on human autoimmune and chronic inflammatory diseases, such as multiple sclerosis and rheumatoid arthritis,5 while little information is available on the role of Th17/Treg imbalance in TB. The studies probing the role of Th17/Treg balance in TB have had various shortcomings. Most of these studies have investigated only one cell subset either Th17 or Treg, which does not allow for a comprehensive evaluation of the Th17/Treg dynamics in TB. Thus, in order to find out whether Th17/Treg balance is successfully maintained in active TB disease, we recruited active TB patients, latent TB subjects, and healthy controls to examine the levels of Th17 and Treg in peripheral blood mononuclear cells (PBMCs) by flow cytometry. In addition, the levels of cytokines, IL-17 and IL-10 related to Th17 and Treg, respectively, were determined in plasma by ELISA.
Materials and methodsStudy subjectsThe study was conducted between January 2013 and July 2015 at Daping Hospital of Third Military Medical University, Chongqing, China. Due approval for the study protocol was obtained from the hospital Ethics Committee. A total of 250 study subjects, including 90 patients with active TB, 80 subjects with latent TB, and 80 healthy controls were enrolled in the study. The active TB patients were diagnosed according to World Health Organization criteria, on the basis of clinical symptoms, and confirmed by chest X-ray radiograph (CXR) and/or positive culture of M. tuberculosis. Latent TB subjects were individuals who had history of close contact with active TB patients, and displayed positive results of interferon-gamma release assay (IGRA) (T-SPOT.TB, from Oxford Immunuotec Ltd, U.K.), but showed no signs or symptoms of active TB disease and had negative cultures for M. tuberculosis. Healthy controls were individuals who had no history of close contact with TB patients, and displayed normal chest radiograph, negative T-SPOT.TB test, and no clinical symptoms of active TB. Written informed consent was obtained from all study subjects. Patients with renal or liver disorders, HIV infection, cancer, autoimmune diseases, and those receiving immunosuppressive therapy (such as glucocorticoids) or having received >7 days of anti-TB therapy were excluded from the study.
T-SPOT.TB assay10mL whole blood from the 250 subjects was obtained. Citrated blood samples were kept at room temperature and the test was carried out on the same day. All participants underwent T-SPOT.TB test in accordance with the manufacturer's instructions. Briefly, the blood samples were separated by density gradient centrifugation to isolate the PBMCs. The PBMC suspension was incubated overnight at 37°C with specific TB antigens, ESAT-6 and CFP-10, in an enzyme-linked immunosorbent spot assay. The spots were counted after enzyme-linked antibody and substrate were added. More than six spots with either antigen were considered as positive, while six or more spots with both antigens were considered as negative. The remaining plasma samples were stored at −80°C before being tested for cytokine levels.
Absolute count of Th17 and Treg cells by flow cytometryAbsolute count of Th17 and Treg cells was performed by flow cytometry. PBMCs were adjusted to the concentrations of more than 1×106cells/L and incubated with various antibodies. Anti-human CD antibodies: CD45-ECD, CD4-FITC, CD3-PC5, CD25-APC, and CD127-PE (Beckman Coulter, USA) were used for Treg detection. Gates were set as high side scatter (SS) and CD45+ cells, followed by CD3+CD4+ cells, CD25+CD127− cells; anti-human CD antibodies: CD45-ECD, CD4-FITC, CD3-PC5 (Beckman Coulter, USA), and IL-17-PE (eBioscience, USA) were used for Th17 detection. Gates were set as high SS and CD45+ cells, followed by CD3+CD4+ cells and CD4+IL-17+ cells. Cells were treated with PerNix-nc kit (Beckman Coulter, USA) before being subjected to Th17 analysis by flow cytometry. All samples were analyzed by Flow Cytometer Navios (Beckman Coulter, USA). The results are expressed as the percentage of Th17 or Treg in PBMCs.
ELISA for plasma IL-10 and IL-17 levelsPlasma concentration of IL-10 or IL-17 was measured using ELISA kits (eBioscience, USA). Briefly, 100μL of plasma or standard solution were added to a 96-well plate, which was coated by anti-human IL-10 or IL-17 antibodies. After addition of biotin-conjugate, the plate was incubated at room temperature for two hours, following which the plate was washed. Working solution of streptavidin-HRP, amplification solution, substrate and stop solution were sequentially added. The optical density at 450nm was measured by microplate reader Wellscan MK3 (Leibo Co., Finland). The results are expressed as pg/mL. The standard concentrations ranged from 0.5pg/mL to 1000pg/mL for IL-17, and from 1.0pg/mL to 1000pg/mL for IL-10. All absorbance values fell within the range of the standard curve.
Statistical analysesNormally distributed variables are reported as mean±standard deviation. Inter-group comparisons were analyzed by one-way analysis of variance (ANOVA) followed by Tukey's post hoc test. p-Value<0.05 was considered as statistically significant. All statistical analyses were performed using SPSS version 19.0 software.
ResultsStudy populationThe characteristics of the study population are summarized in Table 1. Mean age of healthy controls was 38.7±22.4 y (range 16–76 y), with 43 males and 37 females, and mean lymphocyte count of 1.6±0.4109/L. Subjects with latent TB had a mean age of 34.2±15.6 y (range 20–65 y), with 45 males and 35 females, and mean lymphocyte count of 1.5±0.3109/L. The active TB patients had a mean age of 36.6±18.8 y (range 16–68 y), with 50 males and 40 females, and mean lymphocyte count of 1.4±0.6109/L. 39 subjects from active TB group were positive for M. tuberculosis culture. No significant inter-group differences were observed with respect to age, gender, and mean lymphocyte count (p>0.05).
Characteristics of study subjects in 3 groups: control, latent TB, and active TB.
| Group | Control | Latent TB | Active TB |
|---|---|---|---|
| Number (n) | 80 | 80 | 90 |
| Age (y) | 38.7±22.4 | 34.2±15.6 | 36.6±18.8 |
| Gender (F/M) | 37/43 | 35/45 | 40/50 |
| Lymphocytes (109/L) | 1.6±0.4 | 1.5±0.3 | 1.4±0.6 |
| T-SPOT.TB | Negative | Positive | Positive |
Age and lymphocytes are expressed as mean±standard deviation.
TB, tuberculosis; T-SPOT.TB, tuberculosis-specific ELISPOT assay.
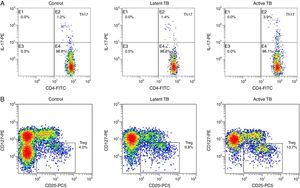
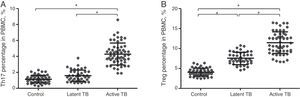
Flow cytometry was performed to evaluate the Th17/Treg dynamics in all subjects. Percentages of Th17 (CD4+IL-17+) and Treg (CD4+CD25+CD127−) in PBMCs from active TB patients, latent TB subjects, and healthy controls (Fig. 1) were determined. The percentages of Th17 and Treg in PBMCs from active TB patients were significantly higher than those from latent TB or control groups (Th17: 4.31±1.35% vs. 1.58±0.71% or 1.15±0.49%, p<0.05; Treg: 11.44±2.69% vs. 7.54±1.56% or 4.10±0.99%, p<0.05). Further, the percentage of Treg was significantly higher in latent TB subjects as compared to that in controls (7.54±1.56% vs. 4.10±0.99%, p<0.05) (Fig. 2).
Representative graphics from flow cytometry for Th17 and Treg measurements. Peripheral blood mononuclear cells were incubated with different antibodies and subjected to flow cytometry analysis. (A) Th17 (CD4+IL-17+) flow cytometry analysis graphics. Th17 level fell in quadrant B2; (B) Treg (CD4+CD25+CD127−) flow cytometry analysis graphics.
Th17 (A) and Treg (B) percentage in PBMCs by study group (control, latent TB and active TB groups) measured by flow cytometry. Results were expressed as mean±SD. Inter-group comparisons were analyzed by one-way ANOVA followed by Tukey's post hoc test. p<0.05 was considered as statistically significant.
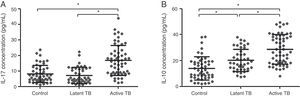
The expression of Th17- and Treg-related cytokines, IL-17 and IL-10, was determined by ELISA in all study groups (Fig. 3). The expression of IL-17 and IL-10 was significantly increased in active TB patients in comparison to that in latent TB or control groups (IL-17: 16.85±9.68 vs. 7.23±5.19 or 8.21±5.51pg/mL, p<0.05; IL-10: 28.70±11.27 vs. 20.25±8.57 or 13.94±9.00pg/mL, p<0.05). No significant difference in IL-17 expression was found between latent TB and control groups (7.23±5.19 vs. 8.21±5.51pg/mL, p>0.05); however, the IL-10 expression was significantly higher in latent TB group as compared to that in the control group (20.25±8.57 vs. 13.94±9.00pg/mL, p<0.05). These observations were consistent with Th17 and Treg results mentioned above.
Expression of cytokines IL-17 (A) and IL-10 (B) in plasma by study group (control, latent TB, and active TB groups), measured by enzyme linked immunosorbent assay (ELISA). Results were expressed as mean±SD. Inter-group comparisons were analyzed by one-way ANOVA followed by Tukey's post hoc test. p<0.05 was considered as statistically significant.
The spectrum of M. tuberculosis infection in humans ranges from latent infection to active disease. The two outcomes of M. tuberculosis infection are often compared to understand the immune response and to study the pathogenesis of tuberculosis. According to this study, we found that the percentage of Treg and Th17 in PBMCs from active TB patients was increased in comparison to that in latent TB or control groups, accompanied by elevated plasma levels of their related cytokines IL-10 and IL-17.
In agreement with the findings of Treg in the present study, Urdahl et al.19 have reported the association between Treg and the severity of M. tuberculosis infection; however, contradictory result has also been observed.20 It is not known whether Treg expansion is the host response to higher levels of inflammation, or if it is the direct consequence of M. tuberculosis infection. The differentiation of Treg after M. tuberculosis infection is perhaps an adaptive immune response aimed at inhibiting inflammation and minimizing the pathological damage induced by an overactive immune response. On the other hand, it is also believed that M. tuberculosis could directly induce the expansion of Treg to facilitate its survival and growth. For example, M. tuberculosis mannose-capped lipoarabinomannan (ManLAM) was found to regulate Treg expansion through prostaglandin E2.14 Trinath et al. further summarized that M. tuberculosis promoted regulatory T-cell expansion via induction of programmed death-ligand 1 (PD-L1) on dendritic cells.21 Most studies have investigated the differentiation of Th17 in PBMCs with or without stimulation by specific TB antigen, and found that the frequency of Th17 decreased in active TB patients compared with that in latent TB subjects.22–24 In the present study, we directly measured Th17 frequency in non-stimulated PBMCs and found the results contrary to our expectations. Nevertheless, our observation is supported by the study from Jurado et al., who demonstrated that the IL-17 over expression in peripheral blood was correlated directly with disease severity.25 Wang et al. reported higher Th17 levels in peripheral blood and pleural fluid of patients with tuberculous pleural effusion. Further, the Th17 levels in peripheral blood were found to be lower than that in pleural fluid samples,26 indicating increased recruitment of Th17 into infected tissues. Thus, it seems plausible that in the initial phase of active TB, most Th17 are recruited to the infected sites, which leads to lower frequency of Th17 in peripheral blood. In addition, with the development of TB disease, large numbers of mature Th17 enter the peripheral blood, which account for the increased Th17 frequency.1 This might explain the contradictory findings in the literature in relation to Th17 levels in TB disease.
Th17 is a relatively newly identified CD4+ T cell subset. The exact role of this IL-17-producing cell in human TB disease has been investigated. A growing number of studies have shown that Th17 is critical in vaccine-induced protection against M. tuberculosis.27,28 However, the role of Th17 in M. tuberculosis infection, other than the recall response remains unclear. In a recent review, Jasenosky et al. summarized that Th17 enhanced the protection against M. tuberculosis most probably at an early stage of infection,1 but not in the late stage. One report showed that in IL-23 subunit p19-deficient mice, there was an almost total loss of Th17, but intriguingly the absence of Th17 had little effect in controlling the infection.29 Given immunopathology as a typical characteristic of M. tuberculosis infection, the role of Th17 in its immunopathogenesis rather than as a protective factor against M. tuberculosis infection might be worthy of research.
The role of Th17 and its secreted cytokine IL-17 in immunopathology is mainly mediated by neutrophils whose influx appears to be associated with aggravation of disease pathology and poor clinical outcomes.30,31 However, the potential mechanism of neutrophil-induced pathological damage is not fully understood. One study showed that neutrophils are the predominant cell types infected with M. tuberculosis in TB patients. Further, they appeared to provide a permissive milieu for a final burst of active replication of M. tuberculosis prior to transmission.32 Apart from recruiting neutrophils, Th17 appears to prolong the survival of these cells,33 and bring about phenotypic changes which render these cells suited to facilitate immunopathogenesis through the production of IL-17.34 IL-17 could also induce tissue damage of tuberculosis patients through promoting the release of proinflammatory cytokines.35,36 Given the role of Th17 and IL-17 as discussed above, regulating the response of Th17 and maintaining the balance between Th17 and Treg is important in TB disease.
In this study, both Treg and Th17 in PBMCs were significantly increased in active TB patients, suggesting that the immunopathology was induced by Th17 as well as the enhanced inhibition of immune response mediated by Treg. However, it is uncertain whether the balance of Th17 and Treg is successfully maintained in the host infected by M. tuberculosis. Various studies have demonstrated increased recruitment of neutrophils to the infection site in experimental animals37,38 and humans32 with active TB disease. It would not be unreasonable to suggest that increased Tregs are unable to fully suppress the immunopathological insult caused by Th17, and the balance of Th17/Treg is altered in the development of TB disease. Further research on mechanisms of the imbalance may help in formulating effective intervention strategies for TB. For instance, as discussed in the Introduction, expression of IL-6 and IL-23 in infected sites could serve to promote the switch of Treg into Th17. Previous studies have also shown that Treg has an inhibitory effect on Th17 differentiation,7 which is probably due to the dependence on the pleiotropic cytokine TGF-beta, secreted by Treg during Th17 differentiation.39
It should be noted that the main limitation of this study is that the results come from measurements obtained only from peripheral blood mononuclear cells (PBMCs) and plasma. Ideally, samples should have been taken from the site where the inflammatory events are occurring either from the alveolar spaces or lung tissue through bronchoalveolar lavage and/or biopsies. In addition, the small sample size is also a limitation. We will pay more attention to these two issues in future studies.
In summary, our study demonstrated an altered balance of Treg/Th17 in active TB patients, with higher percentages of both Th17 and Treg in PBMCs. Further research on this imbalance may offer a new direction for TB treatment.
Conflicts of interestThe authors declare no conflicts of interest.
This work was supported by the National Natural Science Foundation of China (81371760), the National Infection Disease Prevention and Cure Special Project of China (2013ZX10003006-003-002), and the Major Project of the “Twelfth Five-year Plan” for Medical Science Development of PLA (AWS14C003-2).