Immune reconstitution inflammation syndrome typically occurs within days after patients undergo highly active anti-retroviral therapy and is a big hurdle for effective treatment of AIDS patients. In this study, we monitored immune reconstitution inflammation syndrome occurrence in 238 AIDS patients treated with highly active anti-retroviral therapy. Among them, immune reconstitution inflammation syndrome occurred in 47 cases (19.7%). Immune reconstitution inflammation syndrome patients had significantly higher rate of opportunistic infection (p<0.001) and persistently lower CD4+ cell count (p<0.001) compared to the non-immune reconstitution inflammation syndrome patients. In contrast, no significant differences in HIV RNA loads were observed between the immune reconstitution inflammation syndrome group and non-immune reconstitution inflammation syndrome group. These data suggest that a history of opportunistic infection and CD4+ cell counts at baseline may function as risk factors for immune reconstitution inflammation syndrome occurrence in AIDS patients as well as potential prognostic markers. These findings will improve the management of AIDS with highly active anti-retroviral therapy.
Highly active anti-retroviral therapy (HAART) has been widely used to treat AIDS patients since 1995. HAART has been shown to strongly inhibit viral replication, reconstitute immune function, control the incidence of opportunistic infections, and effectively reduce the morbidity and mortality of AIDS patients.1 However, about 10–30% of patients receiving HAART show clinical deterioration featuring increased chances of opportunistic infections and new pathogenic infections, despite increased CD4+ counts and decreased plasma HIV viral loads.2 This phenomenon was described as immune reconstitution disease (IRD) or immune reconstitution inflammatory syndrome (IRIS), which is life-threatening.3
Despite efforts to investigate clinical manifestations of IRIS and involved pathogens, IRIS remains a largely unknown and partially understood condition.4 Publications on this subject are mainly retrospective observational studies, and little is known regarding prognostic factors and risk in different patient populations. In November 2009, Chinese Ministry of Health announced that there were 320,000 HIV-infected patients in China. Among them, more than 80,000 patients have received HAART as the first-line treatment.5 However, the prevalence of IRIS in Chinese AIDS patients receiving HAART, the potential factors which may cause IRIS, and biological markers for predicting IRIS in AIDS have not yet been reported. In this study, we analyzed several factors that may predispose patients receiving HAART to IRIS as well as clinical manifestations in 238 AIDS patients.
Subjects and methodSubjects238 AIDS patients were recruited for the study from October 2007 to September 2009. AIDS was diagnosed by HIV antibody detection at the Center for Disease Control (Hunan Province, China). Patients were informed of the purpose of medication, possible side effects, and signed consent forms were evaluated and approved by Medical Ethics Committee in Second Xiangya Hospital (Hunan Province, China). All treatments used in this study complied with requirements of the National AIDS Free Antiviral Therapy Manuals in China (the second edition). Patients were monitored for 24 weeks. Patient characteristics including gender, age, occupation, possible transmission route, medical history, and the history of opportunistic infection before HAART were recorded.
A prospective observational criterion was followed so that patients who developed IRIS were allocated to the IRIS group (n=47), while the rest was assigned to non-IRIS groups (n=191). Blood samples were collected at baseline, 12th week and 24th week after the initiation of HAART and subjected to a series of tests, such as HIV RNA viral load, CD4+ cell count, biochemical tests, and pathogen detection. Clinical symptoms were detected in a timely manner to guide diagnosis and treatment in practice. In addition, the same tests were performed on 31 healthy volunteers (20 male and 11 female), with an average age of 31.1±5.4 years.3
Instruments and reagentsMonoclonal antibodies including APC-CD4, PE-CD8, FITC-CD3, PerCP-CD45 and disposable hemolysin FACS lysing solution were purchased from BD Biosciences (Shanghai, China). Human HIV-1 fluorescence quantitative polymerase chain reaction diagnostic kits were purchased from Shenzhen PG Biotechnology Company (Shenzhen, China). MK 2 ELISA kits were from Labsystems Dragon (Wellscan, Finland). FACS Calibur flow cytometry was purchased from BD Biosciences. GeneAmp PCR system 5700 was purchased from Applied Biosystems (Carlsbad, CA, USA).
Diagnostic criteria of IRISThere are no universally accepted clinical diagnostic criteria for IRIS currently. However, a tentative definition and diagnostic criteria has been worked out in an international meeting held in Uganda, December 2006, attended by over one hundred experts in this field. IRIS is defined according to the International Network for the Study of HIV-associated IRIS (INSHI).6
Statistical analysisData were analyzed using SPSS (version 13.0) statistical software package and presented as mean values±standard deviation (SD). The comparison of parametric data between groups was performed using t test. Non-parametric data were analyzed using Wilcoxon Rank-sum test. p<0.05 was considered statistically significant.
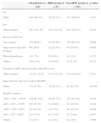
ResultsHistory of opportunistic infections is a risk factor for IRIS during HAARTAmong 238 AIDS patients receiving HAART, 47 cases (19.7%) developed IRIS within 24 weeks after initial HAART administration. There were no significant differences in gender, age, route of infection, duration of infection, or HAART regimens between IRIS patients and non-IRIS patients. However, more patients in the IRIS group had a history of opportunistic infections before the initiation of HAART than in the non-IRIS group (Table 1) (68.1% vs. 22.5%, p<0.001). This suggested that a history of opportunistic infections before HAART is a risk factor for IRIS occurrence during HAART.
Clinicopathological characteristics in patients receiving HAART.
| All patients (n=238) | IRIS group (n=47) | Non-IRIS group (n=191) | p-value | |
|---|---|---|---|---|
| Sex | ||||
| Male | 163 (68.4%) | 32 (68.1%) | 131 (68.6%) | 0.947 |
| Age | ||||
| Mean (range) | 38.3 (30–45) | 37.8 (30–44) | 38.4 (29–47) | 0.435 |
| Routes of infection | ||||
| Sex contact | 150 (63%) | 30 (63.8%) | 130 (68.1%) | 0.580 |
| Intravenous injection of drugs | 69 (29%) | 12 (25.5%) | 47 (24.6%) | 0.895 |
| Blood transfusion | 4 (1.7%) | 0 (0.0%) | 4 (2.1%) | 0.317 |
| Others | 15 (6.3%) | 5 (10.6%) | 10 (5.2%) | 0.172 |
| Duration of HIV infection before HAART (year) | ||||
| Mean (range) | 3.2 (2.1–6.2) | 3.1 (1.9–6.0) | 3.2 (2.0–6.3) | 0.931 |
| Opportunistic infections before HAART | ||||
| Mean | 75 (31.5%) | 32 (68.1%) | 43 (22.5%) | <0.001 |
| HAART regimen | ||||
| AZT+3TC+NVP | 150 (63.0%) | 29 (61.7%) | 121 (63.4%) | 0.834 |
| d4T+3TC+NVP | 36 (15.1%) | 5 (10.6%) | 31 (16.2%) | 0.338 |
| AZT+3TC+EFV | 24 (10.1%) | 4 (8.5%) | 20 (10.5%) | 0.689 |
| d4T+3TC+EFV | 23 (9.7%) | 8 (17.0%) | 15 (7.9%) | 0.057 |
| Others | 5 (2.1%) | 1 (2.1%) | 4 (2.1%) | 0.989 |
HAART, highly active anti-retroviral therapy.
HIV RNA viral load in IRIS group decreased robustly from 4.94 at baseline to 2.82 at the 12th week. Then HIV RNA level further decreased to 2.65 at the 24th week (Table 2). There was no significant difference in the viral load between the two groups at all three time points. No virus rebound in either group was detected during the 24-week follow-up.
Decrease in HIV viral load over time following HAART treatment.
| Unit: Log10 (viral copies/mL) | Non-IRIS group (n=50) | IRIS group (n=47) | p-value |
|---|---|---|---|
| Baseline | |||
| HIV RNA | 5.18±0.59 | 4.94±0.81 | 0.129 |
| Number of samples below LOD | 0 | 0 | |
| 12th week | |||
| HIV RNA | 2.86±0.39 | 2.82±0.43a | 0.764 |
| Number of samples below LOD | 22 (44.0%) | 24 (52.2%) | |
| 24th week | |||
| HIV RNA | 2.63±0.48 | 2.65±0.54a | 0.917 |
| Number of samples below LOD | 47 (94.0%) | 44 (93.6%) | |
HAART, highly active anti-retroviral therapy; LOD, limit of detection.
aNo statistical significance observed.
In the IRIS group, CD4+ cell count increased from 48/μL at baseline to 118/μL at the 12th week, and to 165/μL at the 24th week, respectively. Similarly, CD4+ cell count persistently increased in non-IRIS patients. At all three time points, the differences in the absolute number of CD4+ cells between IRIS and non-IRIS patients are statistically significant (Table 3). These data indicate that patients with low level of baseline CD4+ cell count are more likely to develop IRIS.
Increase in CD4+ T cells over time following HAART treatment.
| Unit: number/μL | Non-IRIS group | IRIS group | p-value |
|---|---|---|---|
| Baseline | n=191 | n=47 | |
| CD4+ cell count | 146±90 | 48±63 | <0.001 |
| 12th week | n=186 | n=46 | |
| CD4+ cell count | 174±116 | 118±62 | 0.019 |
| 24th week | n=175 | n=44 | |
| CD4+ cell count | 269±129 | 165±116 | <0.001 |
HAART, highly active anti-retroviral therapy; IRIS, immune reconstitution inflammation syndrome.
In this study, we identified that a history of opportunistic infections and low CD4+ cell counts before patients receiving HAART are risk factors for IRIS development. To the best of our knowledge, this is the first prospective study to observe IRIS in real time after HAART therapy. Also, this is the first report for the incidence of IRIS in China. Our results showed that 19.7% of AIDS patients developed IRIS, which is consistent with the 10–30% incidence rate reported by other groups.7 No significant differences in age, gender, or route of transmission were observed between IRIS patients and non-IRIS patients (p>0.05). No statistically significant difference was observed between different drug regimens (p>0.05, data not shown).
At present, there is no universal definition for IRIS. Clinicians worldwide follow a set of loose guidelines to determine whether or not a patient developed IRIS such as: appearance of atypical clinical manifestations, aggravation of conditions after HAART initiation, evidence of viral load reduction, as well as immunological recovery (exemplified by the tuberculin skin test turning from negative to positive), clinical manifestations deteriorated due to unknown influences other than drug resistance or side effects, etc.8 In our study, we utilized INSHI criteria for early IRIS diagnosis, which is widely accepted by researchers and clinicians.9 Changes in HIV RNA viral load and CD4+ T cell counts were explicitly requested for the diagnosis of IRIS previously. However, these tests were not done routinely due to the lack of resources, particularly in developing countries. In China, HIV RNA viral load test is only performed every 6 or 12 months after the initiation of HAART, and CD4+ T cells are tested every three months. Therefore, it was difficult to monitor the occurrence of IRIS in a timely manner. In this study, we tried to define clinical symptoms that closely correlate with IRIS to decrease the heavy dependence on laboratory indices.3,10 Other reasons why compulsive testing of HIV RNA viral load and CD4+ T cell counts is not needed include: viral load decreased dramatically in most patients who received HAART regardless of IRIS development11; in some cases, IRIS occurred before the rise of CD4+ T cell count.12 In addition, the number of CD4+ T cells in peripheral blood may not correlate with its function.13 Because of these flaws, CD4+ T cell count has not been considered a necessary parameter for IRIS diagnosis.13
Our study identified two factors which significantly correlated with IRIS occurrence. The first is a history of opportunistic infections before HAART.14,15 A majority of IRIS patients (68.1%) had opportunistic infections before HAART, which is significantly more than non-IRIS patients (22.5%) (p<0.001). Therefore, a history of opportunistic infections may be a risk factor for IRIS. The second factor is CD4+ T cell count at baseline, but not after HAART. It has been shown that patients with more than 350/μL CD4+ T cells are unlikely to develop IRIS. The risk for IRIS increases significantly when CD4+ T cell counts drop below 100/μL.16 Consistently, our results showed that 85.1% of IRIS patients had a CD4+ cell count below 100/μL before HAART, which was 2-fold higher than non-IRIS patients (p<0.001). There may be multiple reasons for this difference in CD4+ cell count: (1) patients with lower CD4+ T cell count are usually at more advanced stages of the disease, and (2) low CD4+ T cell count suggests severe damage to the immune system, and the inability to maintain homeostasis.
Whether HIV RNA viral load is a risk factor for IRIS remains controversial. Shelburne et al. showed that rapid reduction of viral load correlates with higher chance of developing IRIS.17 In contrast, Ratnam et al. study suggested that there is no statistically significant difference in the decrement of HIV RNA viral load between high risk and low risk groups.4 Our data agree with the latter. Although viral load is an informative test, our study could not determine its predictive value.
IRIS is a life-threatening disease for AIDS patients. Mortality rates for IRIS is less than 5% in Europe and North America, and considerably higher in less resourceful districts.12 Life span for IRIS patients vary greatly, depending on the type of pathology, affected organs, etc.18 Close collaboration between clinicians and researchers in our study as well as prompt feedback from laboratory data assured that the best treatment was given to the patients. Only two patients passed away (4.3% mortality rate) during our 6-month study. Clearly, close observation will improve our understanding of IRIS.
Conflict of interestAll authors declare to have no conflict of interest.