Immune response to vaccination in infants born prematurely may be lower than in infants born at full-term. Some clinical factors might be associated with humoral immune response.
ObjectivesThe objectives of this study were to compare the immune response to measles and varicella vaccination in infants born prematurely with those born at full-term and to analyze factors associated with measles and varicella antibody levels.
MethodsProspective study including two groups of infants aged 12 months. One group of infants born prematurely with birth-weight <1500g and who were in follow-up at the outpatient clinic for preterm infants at the institution and other group of infants born at full-term. Infants with malformations, primary immunodeficiency diseases, born to HIV-positive mothers or who had received plasma or immunoglobulin transfusions five months before or three weeks after vaccination were excluded. Plasma antibodies were measured by ELISA and factors associated with antibody levels were assessed by linear regression.
ResultsSixty-five premature and 56 full-term infants were included. The percentage of immune individuals after vaccination against measles (100% vs. 100%) and varicella (92.5% vs. 93.2%) were similar in both groups, as well as the antibody levels against measles (2.393 vs. 2.412UI/mL; p=0.970) and varicella (0.551 vs. 0.399UI/mL; p=0.114). Use of antenatal corticosteroids decreased measles antibody levels whereas breastfeeding for more than six months increased varicella antibody levels.
ConclusionsHumoral responses to measles and varicella were similar between infants born prematurely and full-term infants. Measles antibody levels were negatively associated with antenatal corticosteroid use; varicella antibodies were positively associated with prolonged breastfeeding.
Even when a vaccine-preventable disease is controlled, groups of susceptible individuals can acquire natural infection and spread it to others.1 Among those susceptible are individuals with a decreased immune response to vaccination due to an underlying condition and infants born prematurely. The latter acquire lower levels of antibodies through the placenta and may exhibit a less effective and less durable immune response to vaccination.2 Premature birth emphasizes the immaturity of the innate and adaptive immune systems – both humoral and cellular – compared to full-term newborns.3 Lower levels of Haemophilus influenzae type b, tetanus, pertussis, diphtheria, poliovirus, and hepatitis B antibodies were detected in children born prematurely with extremely low birth weight who were assessed at the age of seven years after booster doses at five years of age.4
Antenatal and postnatal corticosteroid use may interfere with the immune response to vaccination in premature infants.5,6 Other factors associated with prematurity, such as transfusions of blood components, low weight gain during the postnatal period, and low breastfeeding rates may also affect the immune response to vaccination.7,8
Whether specific vaccines would induce a reduced immune response in premature infants, and up to what age that effect would persist still remain unclear.
In this context, the aim of this study was to compare the production of antibodies before and after measles and varicella vaccination in infants born prematurely with very low birth weight and in infants born at full term, and to identify the factors associated with antibody levels against measles and varicella.
MethodsThis was a prospective study conducted from September 2007 to January 2010. The Institution's Ethics Committee approved the project, and the infants’ parents/guardians were asked to sign a statement of informed consent (CEP 0562/09).
The study included two groups of infants aged 12 months who were immunized with measles, mumps, and rubella vaccine (MMR) administered at 12 months of age, according to the Brazilian immunization recommendations.9 Varicella monovalent vaccine (Varilrix, GlaxoSmithKline, Belgium) was administered at 15 months of age. The premature group consisted of infants born at a gestational age of less than 37 weeks and birth-weight of less than 1500g who were in follow up at the Institution's multidisciplinary premature outpatient clinic. The term group consisted of infants born at full-term, adequate for gestational age, with no neonatal clinical complications, discharged from the maternity unit within 2–3 days and followed at a pediatric outpatient clinic.
Infants with congenital malformations, children born to HIV-infected mothers, those with a primary immunodeficiency, infants who received plasma or immunoglobulin transfusion five months before MMR vaccination until last blood collection at 18 months of age or those vaccinated for measles or varicella before the study period were all excluded.10
Mother and child demographic and clinical data were collected from the medical records, and the following information was collected on study inclusion: chronological age, weight, length, body mass index, and clinical complications during the first 12 months of life.11
At 12 months of age, before vaccination against measles, mumps and rubella virus, 4mL of peripheral blood was collected for evaluation of humoral immunity to measles virus vaccine. At 15 months of age, before vaccination against varicella, 4mL of peripheral blood was collected for evaluation of humoral immune response for MMR (post-vaccination dosage) and varicella-zoster virus (pre-vaccination). Finally, at 18 months of age, 4mL of blood was collected for varicella-zoster post-vaccination antibody evaluation.
Measles antibodies were measured by indirect enzyme-linked immunosorbent assay (ELISA), as previously described.12 Individuals with antibody levels ≥0.120IU/mL were considered immune against measles.13
Varicella antibodies were measured by ELISA.14 Individuals with antibody levels ≥0.100IU/mL was considered immune against varicella.15
Statistical analysisNumerical variables were compared using the t-test (normal distribution) or Mann–Whitney U test (non-normal distribution), and categorical variables were compared using the χ2 or Fisher's exact test. Factors associated with post-vaccination antibody levels were analyzed by linear regression. For sample size calculations, groups of 10–20 infants were included for each variable considered in the linear regression model. The Statistical Package for the Social Sciences (SPSS) for Windows v.17.0 (IBM SPSS Statistics, Somers, NY, USA) was used for statistical analysis, and p<0.05 was considered significant.
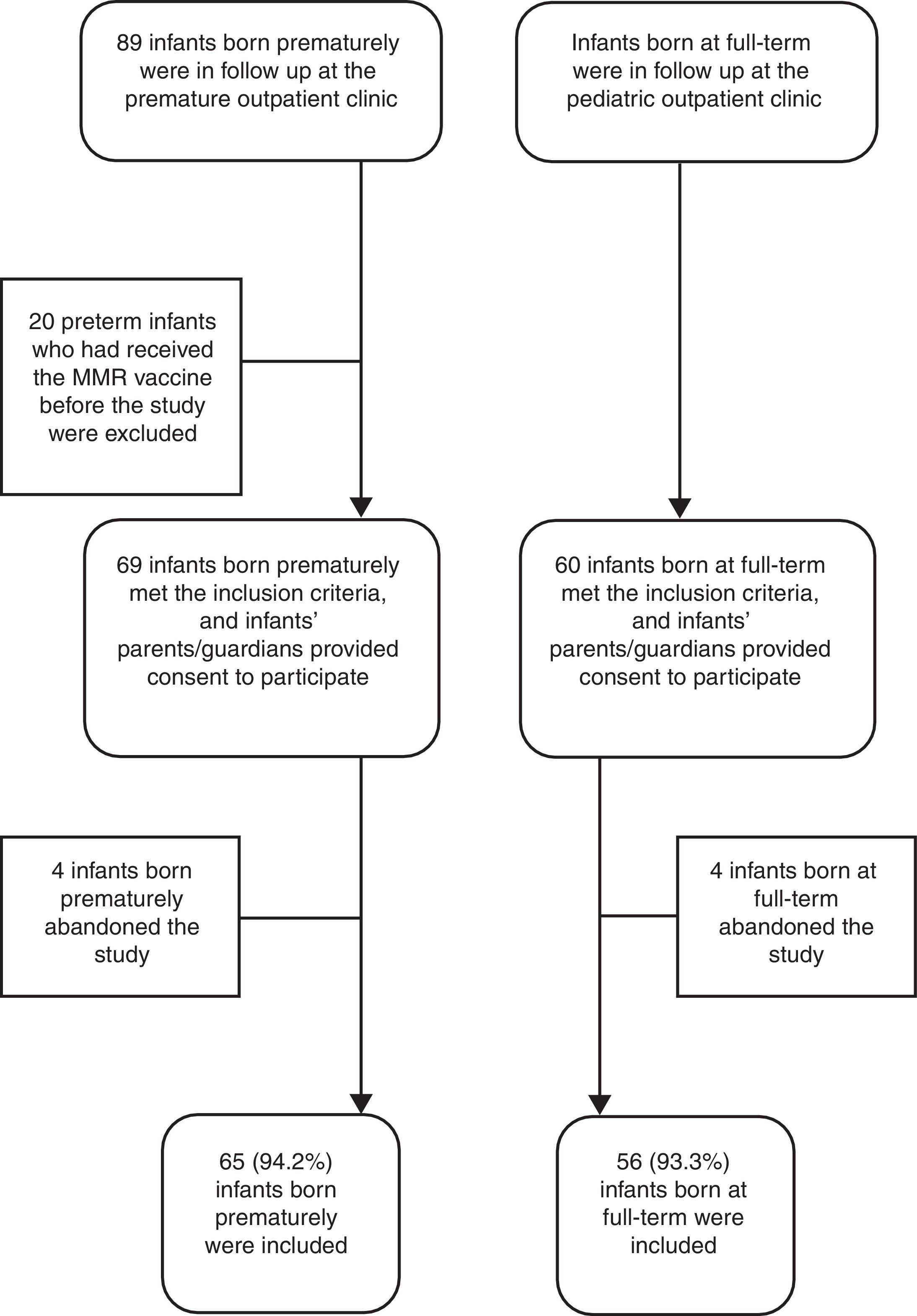
ResultsDuring the study period, among the 89 infants born prematurely in follow up at the premature outpatient clinic of the Institution, 20 had received the MMR vaccine before the study entry and were excluded. Of the 69 infants included, 4 (5.8%) abandoned the study before the collection of blood samples. Therefore, 65 infants born prematurely (25–34.4 weeks of gestation) were compared to 56 infants born at full-term (Figure 1). The comparative analysis for these two groups is shown in Table 1.
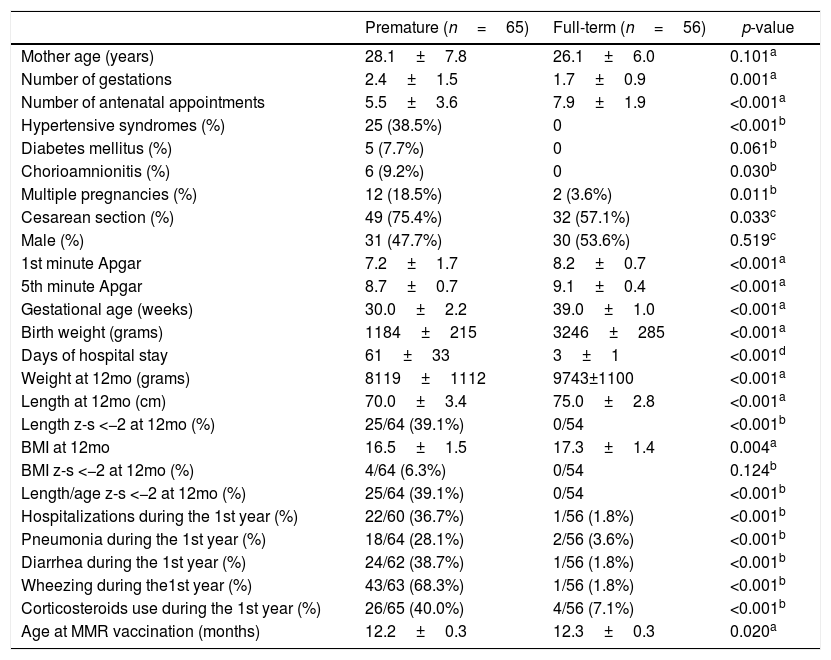
Demographic and clinical characteristics of the studied infants, expressed by mean±SD or number and percentage.
| Premature (n=65) | Full-term (n=56) | p-value | |
|---|---|---|---|
| Mother age (years) | 28.1±7.8 | 26.1±6.0 | 0.101a |
| Number of gestations | 2.4±1.5 | 1.7±0.9 | 0.001a |
| Number of antenatal appointments | 5.5±3.6 | 7.9±1.9 | <0.001a |
| Hypertensive syndromes (%) | 25 (38.5%) | 0 | <0.001b |
| Diabetes mellitus (%) | 5 (7.7%) | 0 | 0.061b |
| Chorioamnionitis (%) | 6 (9.2%) | 0 | 0.030b |
| Multiple pregnancies (%) | 12 (18.5%) | 2 (3.6%) | 0.011b |
| Cesarean section (%) | 49 (75.4%) | 32 (57.1%) | 0.033c |
| Male (%) | 31 (47.7%) | 30 (53.6%) | 0.519c |
| 1st minute Apgar | 7.2±1.7 | 8.2±0.7 | <0.001a |
| 5th minute Apgar | 8.7±0.7 | 9.1±0.4 | <0.001a |
| Gestational age (weeks) | 30.0±2.2 | 39.0±1.0 | <0.001a |
| Birth weight (grams) | 1184±215 | 3246±285 | <0.001a |
| Days of hospital stay | 61±33 | 3±1 | <0.001d |
| Weight at 12mo (grams) | 8119±1112 | 9743±1100 | <0.001a |
| Length at 12mo (cm) | 70.0±3.4 | 75.0±2.8 | <0.001a |
| Length z-s <−2 at 12mo (%) | 25/64 (39.1%) | 0/54 | <0.001b |
| BMI at 12mo | 16.5±1.5 | 17.3±1.4 | 0.004a |
| BMI z-s <−2 at 12mo (%) | 4/64 (6.3%) | 0/54 | 0.124b |
| Length/age z-s <−2 at 12mo (%) | 25/64 (39.1%) | 0/54 | <0.001b |
| Hospitalizations during the 1st year (%) | 22/60 (36.7%) | 1/56 (1.8%) | <0.001b |
| Pneumonia during the 1st year (%) | 18/64 (28.1%) | 2/56 (3.6%) | <0.001b |
| Diarrhea during the 1st year (%) | 24/62 (38.7%) | 1/56 (1.8%) | <0.001b |
| Wheezing during the1st year (%) | 43/63 (68.3%) | 1/56 (1.8%) | <0.001b |
| Corticosteroids use during the 1st year (%) | 26/65 (40.0%) | 4/56 (7.1%) | <0.001b |
| Age at MMR vaccination (months) | 12.2±0.3 | 12.3±0.3 | 0.020a |
z-s, Z score (according WHO, 2007); BMI, body mass index; MMR, measles, mumps and rubella; p:
Of the 65 premature infants, 24 (36.9%) were small for gestational age and 49 (75.4%) were exposed to antenatal corticosteroids. In the neonatal unit, 37 (56.9%) premature infants had respiratory distress syndrome, 17 (26.2%) had patent ductus arteriosus, 29 (44.6%) had clinical sepsis, and 25 (38.5%) had bronchopulmonary dysplasia. During hospitalization, 40 (61.5%) premature infants required mechanical ventilation, 6 (9.4%) received corticosteroids, and 31 (47.7%) received at least one red blood cell transfusion.
The rate of breastfeeding (65.5% vs. 87.5%, p=0.006) was lower and duration (3.2±3.7 vs. 8.9±6.3 months, p<0.001) shorter in premature than term infants. Approximately 15.5% and 57.1% of premature and full-term infants, respectively, were breastfed for >6 months (p<0.001).
Humoral immunityThe percentages of infants with humoral immunity to measles (100% vs. 100%) and varicella (92.5% vs. 93.2%, p=1.00) after vaccination were similar in both groups. No infant had protective measles antibody titers at 12 months, but all showed evidence of humoral immunity three months after vaccination.
At 15 months, nine premature and 11 full-term infants had protective varicella antibody titers before vaccination, likely because they had developed subclinical varicella, and they were excluded from the varicella study. After the exclusion of these 20 infants, the proportion of patients with specific varicella immunity after vaccination assessed at 18 months of age was similar in both groups (92.5% vs. 93.2%, p=1.00).
Both groups of infants showed a significant increase (p<0.001) of antibody titers after measles (premature: 0.025±0.255 vs. 2.393±5.714; term: 0.026±0.261 vs. 2.412±4.095) and varicella (premature: 0.042±0.025 vs. 0.551±0.763; term: 0.039±0.022 vs. 0.399±0.523) vaccination, with a similar humoral immune response following measles and varicella vaccination (Table 2).
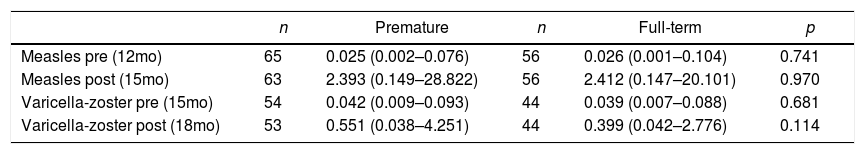
Humoral immunity against measles and varicella-zoster: geometric mean (range) of antibody levels before and after vaccination, expressed in IU/mL.
| n | Premature | n | Full-term | p | |
|---|---|---|---|---|---|
| Measles pre (12mo) | 65 | 0.025 (0.002–0.076) | 56 | 0.026 (0.001–0.104) | 0.741 |
| Measles post (15mo) | 63 | 2.393 (0.149–28.822) | 56 | 2.412 (0.147–20.101) | 0.970 |
| Varicella-zoster pre (15mo) | 54 | 0.042 (0.009–0.093) | 44 | 0.039 (0.007–0.088) | 0.681 |
| Varicella-zoster post (18mo) | 53 | 0.551 (0.038–4.251) | 44 | 0.399 (0.042–2.776) | 0.114 |
p, Mann–Whitney test; Pre, pre-vaccination; post, post-vaccination.
Multiple linear regression analysis was performed to determine an association between demographic/clinical factors and humoral immune response to measles and varicella vaccination. The following independent variables were incorporated into regression models: gestational age, small for gestational age, use of at least one cycle of antenatal corticosteroids, need for at least one red blood cell transfusion, and breastfeeding for more than six months. After adjustment for these variables, the final linear regression model showed that antenatal corticosteroid use reduced the level of measles antibodies by −3.436IU/mL (95% confidence interval [CI]: −6.011 to −0.861, p=0.009), and breastfeeding for more than six months increased antibody levels after varicella vaccination by 0.388 (95% CI: 0.056 to 0.720, p=0.023). Each extra week of gestation reduced measles antibody levels by −0.240 (95% CI: −0.478 to −0.001, p=0.049) and varicella antibody levels by −0.039 (95% CI: −0.075 to −0.005, p=0.027).
DiscussionWe have shown that infants born prematurely and with very low birth weight can have a similar humoral immunity to measles and varicella after vaccination when compared to those born at term.
In the present study, no infant had specific humoral immunity to measles before vaccination at 12 months of age, highlighting the significant portion of susceptible infants at that age. Premature infants may become susceptible to measles at an even earlier age as they have a reduced placental antibody transfer; this has prompted discussions about the ideal age for measles vaccination.16
In this context, a study of premature infants with a mean gestational age of 26.8 weeks and infants born at term showed similar antibody levels and percentage of immune individuals after measles17 and varicella vaccination at 15 months of age.18 It is possible that vaccinations performed after one year of age could justify such a result, as the immune response to vaccination progressively improves with the increase of chronological age.12,19
The beneficial effect of breastfeeding on the production of varicella antibodies was consistent with another study published earlier by our group, showing that breastfeeding for more than six months increased the chance of having complete tetanus immunity after booster vaccination at 15 months of age as well as tetanus antibody levels at 18 months of age.20 Similarly, other studies have shown that higher levels of antibodies were observed after administration of the H. influenzae type b tetanus toxoid conjugate vaccine in infants with longer periods of breastfeeding, as well as a positive correlation between breastfeeding and the CD8+ T-cell population size.21,22
Antenatal corticosteroid use provides numerous benefits to premature infants, but multiple doses have been associated with adverse neurological prognosis; however, its effect on the immune response is controversial.5,6,23 In the present study, we observed a negative effect on the humoral immune response to measles vaccination, similar to that reported after the use of corticosteroids for bronchopulmonary dysplasia treatment.5 However, another study showed no influence on specific cellular immune responses after pertussis vaccination in premature infants.24
Gestational age negatively influenced measles and varicella antibody concentrations. This finding, contrary to our expectations, could result from the inhibitory effect of maternal antibodies in the immune response to vaccination, which would provide an advantage to premature infants by presenting a lower concentration of these antibodies.25 In this sense, Gans et al.26 observed that 95% of full-term breastfed infants who were vaccinated for measles at six months of age showed a cellular and/or humoral immune response.
The strengths of this study were the inclusion of a large number of premature infants and a control group of infants born at term without complications during the neonatal period and after hospital discharge, suggesting an “ideal” control, aside from the small loss of cases.
In conclusion, measles and varicella vaccination administered at 12 and 15 months of age, respectively, resulted in protective antibodies in infants born prematurely with very low birth weight and at comparable levels of those observed among infants born at full-term. Whereas measles antibodies were negatively associated with the use of antenatal corticosteroids, the immune response to varicella was positively associated with breastfeeding for more than six months.
FundingThis work was supported by “Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP),” Brazil (#06/51865-8 and 09/14351-4).
Conflicts of interestThe authors declare no conflicts of interest
The authors acknowledge Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP), Brazil, for research support (#06/51865-8 and #09/14351-4). The authors also acknowledge Juliana Pires and Mônica Lopes for laboratorial analysis of the patients included in the study, and Dra Célia Cristina Pereira Bortolleto from Health Secretary of Suzano Municipality and professionals from Unidade Básica de Saúde Pref. Alberto Nunes Martins, Suzano, for their support.