Brazil is a huge continental country with striking geographic differences which are well illustrated in the HIV/AIDS epidemic. Contrasting with the significant decline in the national AIDS detection rate in the last decade, a linear growth has been reported in the Northern region. Despite its public health and epidemiologic importance, there is scarce HIV-1 molecular data from Northern Brazil. This scoping review summarizes recent epidemiologic data with special emphasis on HIV-1 genetic diversity and antiretroviral drug resistance mutations in patients from the seven Northern states of Brazil. Studies from the Northern Brazil on different HIV-1 genomic regions, mostly pol (protease/reverse transcriptase) sequences of naïve/antiretroviral treated adults/children were retrieved from PubMed/MEDLINE electronic database. These studies indicate a consistent molecular profile largely dominated by HIV-1 subtype B with minor contribution of subtypes F1 and C and infrequent detection of other subtypes (A1, D, K), recombinants (BF1, BC), circulating recombinant forms (CRF) as the new CRF90_BF1 and CRF02_AG-like, CRF28–29_BF-like, CRF31_BC-like, and a potential new CRF_BF1. This pattern indicates a founder effect of subtype B and the introduction of non-B-subtypes and recombinants probably generated in the Southern/Southeastern regions. In naïve populations transmitted drug resistance (TDR) can impact the outcome of first-line antiretroviral treatment and prophylactic/preventive regimens. In the Northern region TDR rates are moderate while patients failing highly active antiretroviral therapy (HAART) showed high prevalence of acquired drug resistance mutations. The limited HIV-1 molecular data from Northern Brazil reflects the great challenges to generate comprehensive scientific data in isolated, underprivileged areas. It also highlights the need to invest in local capacity building which supported by adequate infrastructure and funding can promote robust research activities to help reduce the scientific asymmetries in the Northern region. Currently the impacts of the overwhelming COVID-19 pandemic on the expanding HIV/AIDS epidemic in Northern Brazil deserves to be closely monitored.
Brazil is the largest and the most affected country by the HIV/AIDS pandemic in the Americas and within its continental territorial dimension, striking socio-economic and cultural contrasts are seen and this multiplicity is also reflected in the Brazilian HIV/AIDS epidemic. According to official data, from 1980 until June 2020 1,011,617 AIDS cases have been reported in Brazil1. In the last decade the national detection rate of AIDS cases declined 17.2% changing from 21.5/100,000 inhabitants in 2009 to 17.8/100,000 in 2019.1 The incidence of AIDS cases in the last decade decreased 33.6% in the Brazilian HIV/AIDS epicenter, the Southeastern region.1 In contrast, during the same period, the Northern region has reported a linear growth with a significant increase in AIDS incidence.1 In Brazil the Southeastern region concentrates 51% of AIDS cases, while the Northern region accounts for 6.7% of the total number of cases.1
The Northern Brazilian region is characterized by a vast territorial area (3,853,676 km²) representing 45% of the national territory and it comprises seven states: Acre/AC (capital: Rio Branco), Amapá/AP (capital: Macapá), Amazonas/AM (capital: Manaus), Pará/PA (capital: Belém), Rondônia/RO (capital: Porto Velho), Roraima/RR (capital: Boa Vista) and Tocantins/TO (capital: Palmas).2 Because of the hardly penetrable rain forest, the Northern region is the least populated, containing 8.8% (18,672,591 inhabitants) of the total Brazilian population of 211,755,692.2 Pará state is the most populated and Roraima is the least inhabited state in the region.2 The Northern region is geographically isolated, remote and has very limited highway connectivity with the more populous and industrialized regions of the country. Adding more complexity to this scenario, the Northern region borders several South American countries (Bolivia, Peru, Colombia, Venezuela, Guyana, Suriname and French Guiana). The limited travel control in this large international border creates the opportunity for foreign HIV/AIDS patients from neighboring countries, with less structured public health services, to seek for specialized medical assistance and AIDS treatment in Northern Brazilian states.
The alarming situation of HIV/AIDS pandemic in Northern Brazil is very well illustrated by recent official data showing that, from 2009 to 2019, AIDS detection rate increased in five of its seven states: Acre (61%), Pará (46.5%) Amapá (21.2%), Roraima (11.9%) and Amazonas (9.8%).1 According to this AIDS incidence data, Roraima ranks first (40.1/100,000 inhabitants), Amazonas is the second (34.8/100,0000) and Pará ranks fourth (27.4/100,000), well above to the national rate (17.8/100,000).1 From 2009 to 2019, the national standardized coefficient of AIDS-related mortality decreased 29.3%, declining from 5.8 to 4.1 deaths/100,000 inhabitants.1 This decline was reported in most Brazilian states, except in six, three of them located in the Northern region (Pará, Amapá and Acre).1 In 2019, four out of eleven states with mortality coefficients above the national rate were located in the Northern region: Pará (7.7 deaths/100,000 inhabitants), Amazonas (6.4/100,000), Amapá (5.8/100,000) and Roraima (5.8/100,000).1 Of remark is the significant increase in the AIDS-mortality coefficient reported between 2009 and 2019 in Amapá and Acre states, increasing from 0.6 to 5.8 and from 1.1 to 2.2 deaths/100,000 inhabitants, respectively.1
Currently, as part of the “Test and Treat policy” adopted by the Brazilian Ministry of Health, over 600,000 patients are under highly active antiretroviral treatment (HAART) at specialized AIDS clinics.1 Transmitted drug resistance (TDR) can lead to first-line antiretroviral (ARV) virological failure, highlighting the need to monitor TDR rates in antiretroviral treatment (ART) naïve patients. TDR can also impact the outcome of other interventions such as prevention of mother-to-child transmission (PMTCT), pre-exposure prophylaxis (PrEP) and post-exposure prophylaxis (PEP).3,4 Besides several Brazilian reports on regional rates of TDR and drug resistance mutations, three official reports by the National Network for Drug Resistance Surveillance (HIV-BResNet) have monitored the transmission of drug-resistant strains to nucleoside and non-nucleoside reverse transcriptase inhibitors (NRTI and NNRTI) and to protease inhibitors (PI) and the HIV subtype distribution in the country.5–7
The molecular epidemiology of the Brazilian HIV-1/AIDS epidemic is complex and dynamic characterized by the co-circulation of several “pure” group M subtypes, especially the predominant subtype B, except in the Southern region where the subtype C is the most prevalent.8–15 In Brazil, the subtype B pandemic lineages (BPANDEMIC) prevail compared to the Caribbean non-pandemic subtype B clades (BCAR), except in some Northern states.16 An increase of non-subtype B infections, mainly subtype C, has been reported, while “pure” subtype F1 remains infrequent, except in the Northeastern region.17,18 HIV-1 full genome/near full genome sequencing studies have shown a growing number of BF1 and BC recombinants which have been classified as circulating recombinant forms (CRFs) or unique recombinant forms (URFs).17,19,20
Despite the epidemiologic and public health importance of the rampant HIV-1/AIDS epidemic in the multifaceted and complex scenario of the Northern Brazil, regional molecular data, including HIV-1 subtype distribution and the prevalence of ARV drug resistance mutations remain poorly described, compared to other Brazilian regions.8,21–33 This scoping review34 summarizes recent epidemiologic data and emphasizes HIV-1 genetic diversity and antiretroviral drug resistance mutations in the seven states of the Northern region, starting from the most affected by HIV-1/AIDS (Pará state) to the least affected (Acre state). Except for one,24 the studies included were identified by a search (March 2020-March 2021) in the PubMed/MEDLINE (biomedical sciences) electronic database (MeSH Terms: HIV-1; HIV AIDS, Northern; Brazil; HIV genetic diversity; HIV antiretroviral drug resistance; HIV subtype diversity; HIV drug resistance mutations), selecting articles from Northern Brazil published in English, from all years, any type of study design, with any given number of patients, of any age and sex, both untreated, under prophylaxis, under treatment and in HAART failure. To better illustrate the socio-economic features of each state, we have included the recent rank of the human development index (HDI), which summarizes key dimensions of human development including life expectancy, education and per capita income (HDI ranges from 0 to 1 and in 2020 the HDI of Brazil was 0.765)2.
HIV-1 genetic diversity and drug resistance mutations in northern Brazilian statesPará stateIn the Northern region, Pará is the most populated and the most affected state by HIV/AIDS (territorial area: 1245,870 km2; population: 8,690,745: demographic density: 6.07 inhabitants/km2; HDI: 0.646).2 From 1980 to 2020 a total of 30,715 AIDS cases have been reported in Pará representing 46.5% increase from 2009 to 2019 (AIDS detection rate in 2009: 18.7/100,000 inhabitants, in 2019: 27.4/100,000).1 From 2009–2019, the coefficient rate of AIDS related mortality in Pará state increased 26.2%.1
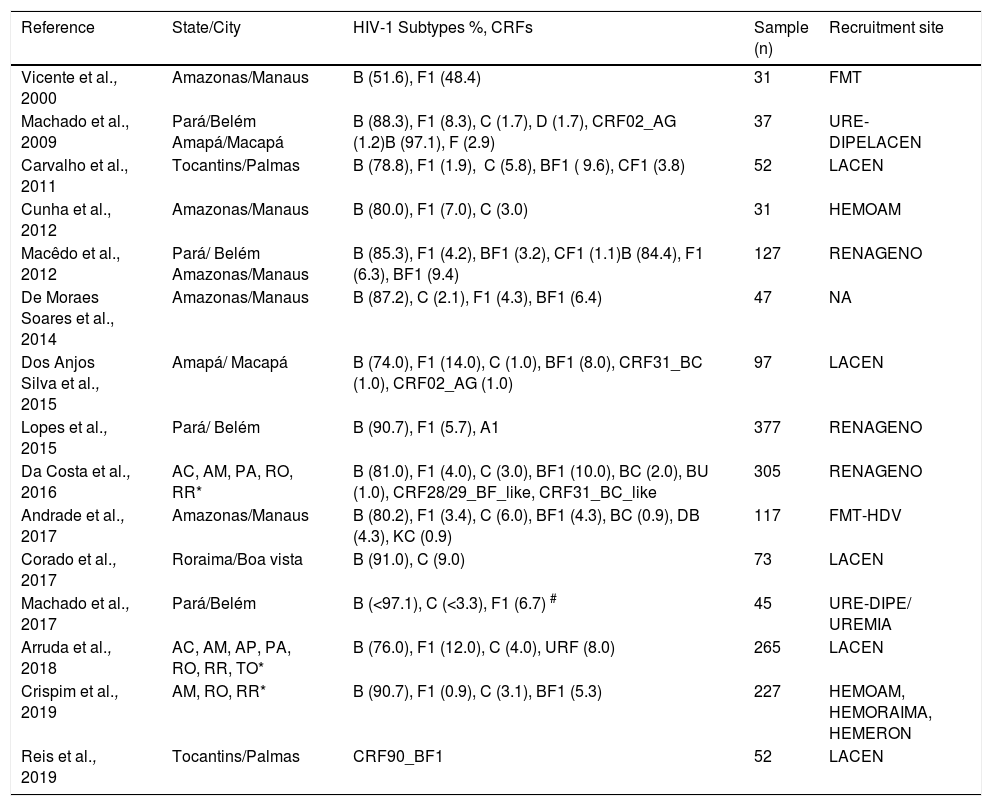
A limited number of samples from Pará was analyzed in the first two reports of the HIV-BResNet; however results of HIV-1 genetic diversity in this state were not disclosed.5,6 The first description of HIV-1 subtypes circulating in Pará reported high subtype B rates (>85%), moderate rate (<10%) of subtype F1, and low rate (<5%) subtype D in both protease (PR) and envelope (ENV) regions. Subtype C rate was low in ENV sequences and in PR sequences, the CRF02_AG was low.21 This study also described for the first time in the Northern region the circulation of both subtype D as mosaics, and of the introduction of the CRF02_AG-like, which is the most worldwide disseminated and highly prevalent CRF in several African countries.35 Subsequently, a study from 2012 confirmed the high prevalence of subtype B (>85%) with minor participation of “non-B” subtypes: <5% for subtype F1 and recombinants (BF1, CF1).24 A following cross-sectional molecular study published in 2015 corroborated the high prevalence of subtype B (>90%) and the marginal contribution of subtypes F1 and C (<1%). This investigation reported also the first case of subtype A1 infection in Pará.27 A comprehensive molecular study in five states of the Northern region (AC, AM, PA, RO, RR) published in 201628 showed that among samples from Pará state, subtype B was highly predominant (>85%) while subtype F1, subtype C and BF recombinants represented ≤5%. In 2017, a study conducted in HIV-1 infected pregnant women confirmed in PR and reverse transcriptase (RT) regions the high predominance of subtype B (>90%) and a minimal contribution of subtype C (≤3%) while subtype F1 was only detected in RT sequences.31 Main data from these studies21,24,27,28,31 are summarized in Table 1 and methods are presented in the supplementary Table.
HIV-1 Subtypes and CRFs described in patients from the Northern Region (2000–2019).
*Initials of Northern states AC: Acre , AM: Amazonas, AP: Amapa, PA: Pará, RO: Rondônia, RR: Roraima, TO: Tocantins. CRFs: Circulating recombinant forms; FMT: Fundação de Medicina Tropical de Manaus; HEMOAM: Fundação de Hematologia e Hemoterapia do Amazonas, do Manaus; LACEN: Laboratório Central; URE-DIPE: Unidade de Referência de Doenças Infecciosas e Parasitárias de Belém-Pará; UREMIA: Unidade de Referência Materno Infantil e Adolescente de Belém-Pará; RENAGENO Rede nacional padronizada para genotipagem do HIV; HEMORAIMA: Hemocentro de Roraima, Boa Vista; FHEMERON: Fundação Hematologia e Hemoterapia de Rondônia, Porto Velho. PMCT – Prevention of mother-to-child transmission. (#) two regions studied: subtype B <97.1% (PR), 90.0% (TR) subtype C <3.3% (TR), 2.9% (PR).
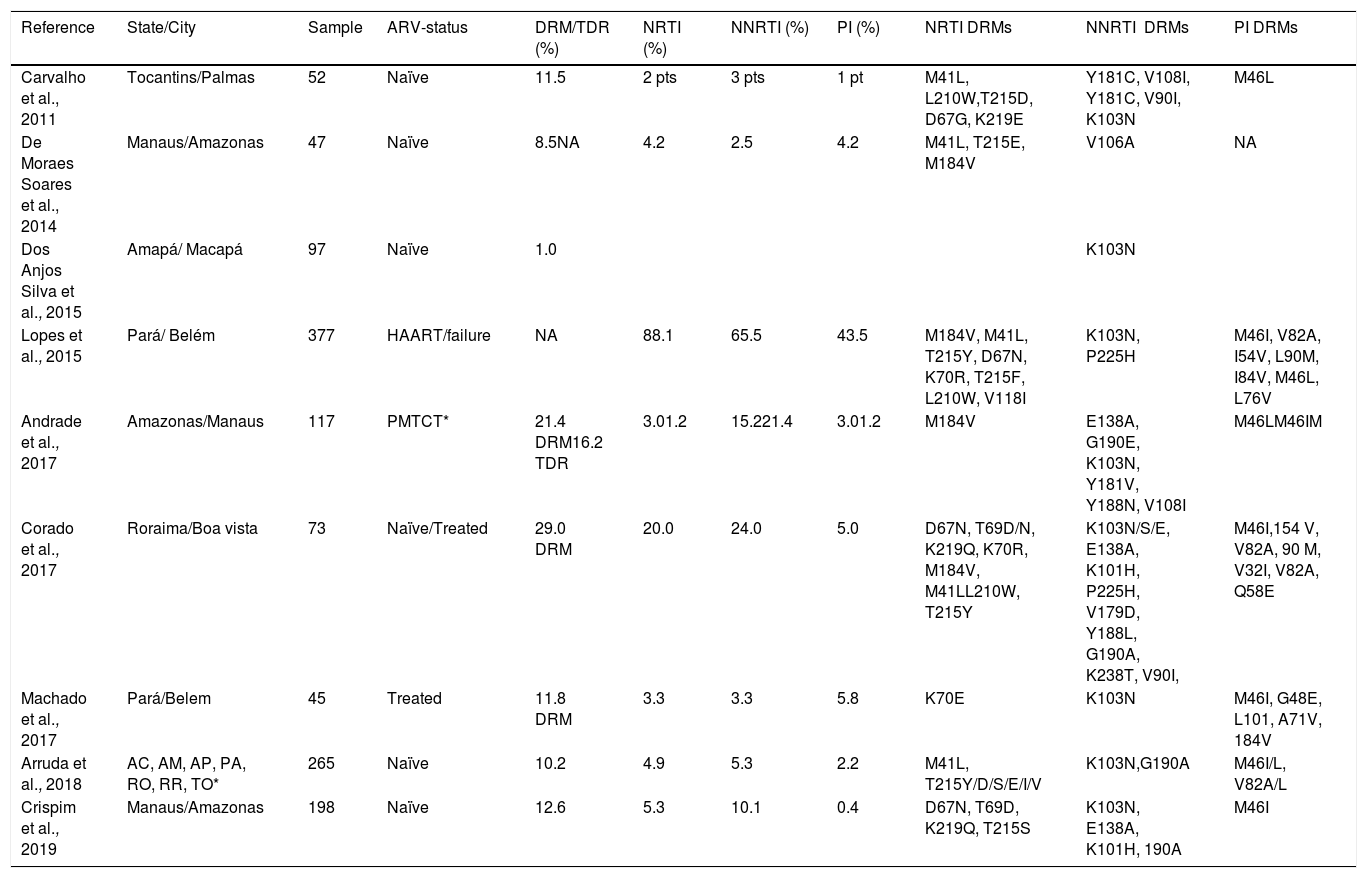
The first report on acquired drug resistance mutations in patients failing HAART from Pará showed high rates (15%) of resistance mutations to NRTIs, NNRTIs and PIs24. ARV resistance profiles in HIV-1 infected pregnant women on ART showed low levels (<5%) of NNRTI and NRTI mutations and around 6% PI mutations.31 In the last HIV-BResNet survey the analyses of PR/RT sequences from Pará showed moderate prevalence of any surveillance drug resistance mutation (SDRM)7. Main class specific drug-resistance mutations identified in these studies7,24,31 are displayed in Table 2.
Prevalence of transmitted drug resistance/TDR and drug resistance mutations/DRMs and main antiretroviral drug resistance mutations to different drug classes in patients infected with HIV-1 from Northern Brazil.
*Initials of Northern states AC: Acre , AM: Amazonas, AP: Amapá"?>, PA: Pará, RO: Rondônia, RR: Roraima, TO: Tocantins., Porto Velho. *PMCT Prevention of mother-to-child transmission. TDR: transmitted drug resistance. NA: not available. De Moraes Soares et al. 2014 reported the general prevalence of mutations. Carvalho et al. 2011, reported the number of patients with specific resistance mutations (patient=pt).
Remarks: Considering all five investigations on HIV-1 genetic diversity conducted in distinct populations from Pará state (adult males and females, including pregnant women) analyzing different HIV-1 genomic regions (ENV, PR, RT) high prevalence of subtype B was reported (> 85%) with low to moderate participation of subtype F1 (<10%) and infrequent and very low detection of subtype C (<5%).21,24,27,28,31 Other “non-B” subtypes as subtype D and A1 and intersubtype recombinants such as BF1, CF1 and the CRF02_AG-like were sporadically identified (Supplementary Figure). The possible influence of domestic and foreign tourism and other local factors in the introduction and dissemination of new variants of HIV-1 in Pará state, remains elusive. On the contrary, the link between the epidemic in Pará state and the Southern/Southeastern regions, suggested by the circulation of several intersubtype recombinant forms, can be supported by ongoing travel and bidirectional migration flows. One study in patients failing HAART showed high rate of ADRM while low ADRM was reported in pregnant women on ART and in ARV-naïve patients, moderate rate of any SDRM was found.7,24,31 Further investigations are necessary to better characterize the rate of ADRM in patients failing HAART and TDR rates among ARV naïve patients living in Pará state which are still very limited.
Amazonas stateThe Amazonas is the largest state in area and the second most affected by HIV/AIDS in the Northern region (territorial area: 1559,167 km²; population: 4207,714 inhabitants; demographic density: 2.23 inhabitants/km², HDI 0.674).2 According to official data 20,196 AIDS cases were reported in Amazonas state from 1980 to 2020 and in 2019 AIDS detection rate was the second highest in the country (34.8/100,000 compared to 17.8/100,000 in Brazil); however the coefficient rate of mortality decreased 4.5% from 2009 to 2019.1
The pioneering study about the HIV-1 genetic diversity in Amazonas state and also in Northern Brazil published in 2000 showed in ENV sequences, similar prevalence of subtypes F1 and B (~ 50%) and the combination of subtypes in ENV and PR regions indicated several different B, C and F recombinants.8 More than a decade later, two studies reported HIV-1 subtypes in PR/RT sequences from Amazonas state.23,24 In seropositive blood donors from the largest public blood bank in Amazonas (Fundação de Hematologia e Hemoterapia do Amazonas, HEMOAM, Manaus) high prevalence of subtype B (>90%), low to moderate rates (>5%- ≤15%) of subtype C and low frequency (<5%) of BF1 recombinant were detected. The subtype C isolates, identified for the first time in Amazonas were phylogenetically related to the Southern region.23 Another study showed high predominance of subtype B (>80%) while subtype F1 and BF1 recombinants represented <10%.24 Main details from these studies23,24 are displayed in Table 1 and in the Supplementary Table.
A national survey from 2014 in which the Northern region was represented by patients from Manaus showed high prevalence of subtype B (>85%), low rates (<5%) of subtypes F1 and C and moderate rate of BF1 recombinants (<15%).25 A robust molecular study from five Northern region states reported in samples from Amazonas, high prevalence of subtype B (>80%), moderate prevalence of BF1 recombinants (<15%) and low rates (<5%) of subtypes C, F1, BC recombinant and other subtypes.28 In 2017, a retrospective cohort of HIV-infected children, born to HIV-infected mothers in Manaus, showed that subtype B represented >80%, a low rate of subtype F1 (<5%) and moderate prevalence of subtype C and URFs (BF1, DB, BC and KC) [>5% - ≤15%] were reported.29 Main results and study details 26,28 are shown in Table 1 and in the Supplementary Table.
A comparative analysis of subtype B sequences collected in 21 Brazilian states from the five geographic regions showed the predominance of BPANDEMIC lineages and <10% BCAR clades; however, in Amazonas state BCAR lineages represented 14%.16 A recent study from 2019 in an important sentinel population for molecular studies (apparently healthy, adult blood donors, recently diagnosed with HIV-1), included participants from three Northern reference public blood banks.32 Among participants from HEMOAM, located in Manaus, subtype B was highly prevalent (>85%), subtypes C and F1 had low prevalence (<5%) while BF1 recombinants were found at moderate rate (≤15%). Around 20% of subtype B sequences from Amazonas belonged to the BCAR lineage. The subtype C and F1 sequences identified in Amazonas clustered within Brazilian C and F1 lineages, respectively. Twelve BF1 mosaics found in Amazonas showed 11 different recombination profiles including singleton URFs, CRF28/29_BF-like and BF1 isolates that branched with other previously described Brazilian BF1 viruses, suggesting a putative new CRF_BF1 originated in the Northern Brazil.32 For further study details, see Table 1 and the Supplementary Table.
A national survey on TDR rates in states from all Brazilian regions, which included ARV naïve patients from Manaus/AM, showed moderate TDR prevalence, similar to the rate observed in the Southern region, but lower than the rate detected in the Northeastern, Southeastern and Central Western regions.25 Sequences identified in a study with children born to HIV-infected mothers in Manaus obtained prior to ART included almost one third of children that had been exposed to ART as part of the prevention of mother-to-child transmission protocol (PMTCT). In this group, one third of mothers received ART during pregnancy, around half had a history of intrapartum zidovudine prophylaxis, and their children received postnatal zidovudine prophylaxis.29 Around one fifth of children had drug resistance mutations (DRM) and in both PMTCT-exposed and unexposed children, NRTI and PI mutations were low (<5%) and NNRTI mutations were high (>15%). The overall TDR prevalence in this cohort was high (>15%): 14.3% in PMTC-unexposed children and 21.1% in PMTCT-exposed ones.29 Main drug resistance mutations are depicted in Table 2.
The most recent HIV-BResNet survey showed in samples from Amazonas moderate SDRM rate (<15%).7 In newly diagnosed ARV naïve blood donors from HEMOAM/Amazonas moderate TDR prevalence was observed.33 In these patients NNRTI mutations predominated at moderate rate and were followed by NRTI mutations and a low rate of PI mutations. Dual-class NNRTI/NRTI mutations were low. Most donors with TDR had multiple and similar drug resistance mutations and highly supported subtype B monophyletic clades of viruses with TDR mutations were identified. The largest transmission TDR cluster of 10 sequences, eight from Amazonas, shared several NRTI and NNRTI mutations.33 The summary of resistance mutations identified7,33 is shown in Table 2.
Remarks: Earliest molecular findings of HIV-1 in Amazonas two decades ago showed similar prevalence of subtypes B and F1 and a higher rate of subtype F1, compared to its low prevalence in Southern Brazil, raising the hypothesis of a founder effect of subtype F and its dissemination southwards.8 However, further HIV-1 molecular studies on different genomic regions (ENV, GAG, POL), mostly on POL, published overtime in different study groups of adults (males, females, ARV naïve,initiating HAART and HAART failure) and children, showed an epidemic highly dominated by subtype B (≥80%).8,23,24,25,28,32 Low to moderate prevalence of subtype F1 and subtype C viruses clustering within Brazilian F1BR and CBR lineages, respectively, indicated their dissemination northwards. Also variable rates of BF1 recombinants (≤10%), including a potential, yet uncharacterized CRF_BF1 originated in Northern Brazil and infrequent BC recombinants were found in Amazonas. A strong epidemiological link with the epidemic in the Caribbean is suggested by the finding of higher prevalence of subtype B sequences belonging to BCAR lineages (Supplementary Figure).16,32 Overall HIV-1 molecular data indicate the need of surveillance studies to monitor HIV-1 diversity in Amazonas including the characterization of full/near-full genome sequences of recombinant forms, which is necessary to fully describe new CRFs.
In Amazonas, PMTCT-exposed and unexposed children born to HIV-1 infected mothers showed a high prevalence of DRM and TDR, respectively.29 In ARV naïve adults, moderate rate of TDR has been reported.7,25,33 The identification of transmission clusters of multidrug-resistant viruses in ARV-naïve blood donors from Amazonas33 highlights the importance of continued monitoring of transmission of ARV resistant viruses which can deteriorate the epidemiologic situation by jeopardizing first-line ARV treatment options. Also, further studies are needed to indicate if these clusters are new emerging TDR/DRM clusters of larger size or if they represent clusters limited to a group of closely related individuals.
Rondônia stateThe Rondônia state (territorial area: 237,765 km2; population: 1796,460 inhabitants; demographic density: 6.58 inhabitants/km2; HDI 0.690),2 ranks third in the number of AIDS cases in the Northern region comprising 6576 reported cases from 1980 to 2020, representing 3.8% reduction from 2009 to 20191. In 2019, the AIDS detection rate in Rondônia was the same as the national rate (17.8/100,000 inhabitants) and from 2009 to 2019, Rondônia state reported 3.8% decrease in AIDS-related mortality.1
A large molecular study on PR/RT sequences of patients failing ART from the Northern region that included sequences from Rondônia28 showed predominance of subtype B (60%) and a high rate (40%) of “non-B” subtypes C and F1. Also a high rate of BF recombinants (>15%) and a moderate rate of BC recombinants (<10%) were reported. A recent study from 2019, that included blood donors from HEMERON/Porto Velho, Rondônia, reported a more homogeneous HIV-1 molecular pattern of highly prevalent subtype B (90%) and 10% subtype C.32 The most recent HIV-BResNet survey that included samples from the Northern region, 29 of them from Rondônia, showed moderate SDRM (<15%).7 Recently, a study with 20 PR/RT sequences of newly diagnosed HIV-1 infected blood donors from HEMERON blood bank did not detect any sequence with mutation associated with TDR.33 Further details of these studies28,32 are presented in Table 1and in the supplementary Table while data on drug resistance mutations7,33 are listed in Table 2.
Remarks: Only two HIV-1 molecular studies performed in patients from Rondônia showed divergent genetic patterns in which although subtype B predominated (60–90%), other variants, in especial subtype C, was found to circulate at low to moderate rates and subtype F1 was either undetected or identified at moderate rate. BF1 recombinants were either undetected or detected at high rate (>15%) and BC recombinants were undetected or found at moderate rate (Supplementary Figure). The findings of significant rates of subtype C and BF1, BC recombinants suggest a connection between the HIV/AIDS epidemics from RO and the Southeastern and Southern region. This assumption can be supported by the influx of Southerners to Rondônia, motivated by cattle farms and agriculture that took place in the 70′s and 80′s. So far, only two studies reported very different rates of ARV drug resistance mutations in patients from Rondônia ranging from undetected to moderate rate, therefore, the actual prevalence of ADRM and TDR in this state, remains to be demonstrated.
Tocantins stateThe Tocantins state was founded in 1989 attracting individuals from all over the country (territorial area: 277,466 km2; population 1590,248, demographic density 4.98 inhabitants/km2, HDI 0.699).2 From 1980–2020 Tocantins state reported 3356 AIDS cases and from 2009 to 2019, 10.3% decrease in the detection rate, together with a decline in the coefficient of AIDS-related mortality were reported.1
The only description of HIV-1 subtypes circulating in patients from Tocantins published in 2011, based on PR/RT sequences of ARV naïve patients, revealed high rate of subtype B (~80%), moderate level of subtype C, and low level of subtype F122. Moderate rate of recombinant viruses was found (<15%) including mostly BF1 (~10%) and CF1 (<5%) (Table 1, Supplementary Table). In these patients, TDR rate was moderate (<15%) and the detected mutations were associated with only one class of ARV drug: moderate frequency of NNRTI mutations and low rate of NRTI mutations and PI mutations (Table 2). The last HIV-BResNet survey which included 21 sequences from Tocantins state did not show individual data on HIV-1 subtypes, while moderate prevalence (<15%) of any SDRM was repoted7 (Table 1, Supplementary Table).
A further molecular study that investigated the evolutionary relationship and mosaic structure of BF1 PR//RT sequences identified in over 800 patients from three Brazilian geographic regions,19 included the BF1 mosaics originally identified in Tocantins state.22 Phylogenetic and boot scan analyses identified clusters with similar recombination points and HIV-1 full/near full length genomes (FLG/NFLG) and partial genomes (cluster #5) from epidemiologically unlinked individuals living in the Northern (Tocantins state) and in the Central Western regions allowed the characterization of a new CRF.19 This recombinant was named CRF90_BF1 representing the 9th CRF_BF1 described in Brazil and the first one described in the Northern (Tocantins state) and Central Western regions.19 Further details of results and methodology of these studies22,19 are shown in Table 1 and in the Supplementary Table.
Remarks: The only report on HIV-1 subtypes circulating in Tocantins from 2011 showed the predominance of subtype B, low rates of subtypes C and F1 (Supplementary Figure). The circulation of diverse intersubtype recombinant forms in TO state suggested an epidemiologic link with the Southern and the Southeastern regions, which can be corroborated by the high influx of migrants to the newly founded Brazilian state three decades ago. Also a full BF1 genome sequence from a patient living in Tocantins contributed to the characterization of the newly described CRF 90_BF1.19 The two reports on drug resistance mutations in TO show moderate levels of TDR and SDRM; however these studies are still limited by the small sample sizes.7,22 The clear shortage of molecular data on both the HIV-1 genetic diversity and drug resistance mutations in patients from TO state demonstrate the importance of efforts to pursue molecular characterization and surveillance studies in this setting.
Roraima stateRoraima is the northernmost state (territorial area 223,644 km2; population 631,181 inhabitants; population density 2.01 inhabitants/km2; HDI 0.707).2 From 1980–2020 a total of 2898 AIDS cases was reported in RR.1 In 2019, the official AIDS detection rate in Roraima was the highest in the country: 40.1/100,000 inhabitants (compared to 17.8/100,000 in Brazil).1 In Roraima, the coefficient of AIDS related mortality in 2019 ranked amongst the highest in the country (5.8 deaths/100,000 inhabitants compared to the national rate of 4.1/100,000).1
A robust investigation on HIV-1 subtypes published in 2016, analyzed pol sequences from the Northern region including 36 sequences from Roraima, showing that subtype B was highly prevalent (>85%) and BF1 recombinants were found at moderate rate (<15%).28 A further study from 2017 in patients from Roraima that included participants from Venezuela and Guyana, showed high prevalence of subtype B (>90%) and moderate rate of subtype C (<10%).30 The last HIV-BResNet survey from 2018, included 12 sequences from Roraima but no individual data on HIV-1 subtypes was shown.7 A recent investigation from 2019 that analyzed Northern HIV-1 infected blood donors, included nine sequences from Roraima and all were subtype B.32 Study details7,32 are presented in Table 1 and in the Supplementary Table.
The most comprehensive study on resistance mutations in Roraima described TDR and ADRM rates in sequences of ARV-naïve and ARV-experienced patients.30 Among a minor group of ARV-naïve patients, one had NNRTI mutation while NRTI or PI mutations were not detected. In a larger group of ARV-experienced patients, around one third had resistance mutations to NRTI and NNRTI, NRTI and/or PI. NNRTI mutations predominated and were followed by NRTI mutations while PI mutations were rare. The most recent HIV-BResNet survey included 12 sequences from Roraima and found moderate SDRM, which was detected in one patient.7 The recent study in newly diagnosed blood donors from the Northern region included nine participants from Roraima and no mutation associated with TDR was detected.33 Results of these studies7,30,33 are summarized in Table 2.
Remarks: Overall the three studies that detailed HIV-1 subtypes circulating in adult patients from Roraima28,30,33 showed an epidemic highly dominated by subtype B infections (>85%), subtype C was either undetected or lower than 10% and BF1 recombinants were undetected or found at moderate rate (Supplementary Figure). Also, based on results from a limited number of ARV-naïve patients (n = 33),7,30,33 the prevalence of TDR and SDRM was moderate while in ARV-experienced patients the rate of ADRM was high. This restricted number of HIV-1 molecular studies from Roraima indicates the necessity of continued studies on HIV-1 subtypes and DRM in both ARV-naive and experienced patients, since Roraima represents an important gateway for Latin American immigrants into Brazil.
Amapá stateThe Amapá state (territorial area: 142,470 km2, population: 861,773 inhabitants; population density: 4.69 inhabitants/ km2, HDI 0.708) has registered 2847 AIDS-cases from 1980 to 2020.1 In 2018, the AIDS detection rate in Amapá (22.3/100,000 inhabitants) ranked amongst the highest in the country (Brazil: 17.8/100,000).1 Between 2009–2019, AIDS mortality coefficient rate increased from 0.6 to 5.8 deaths/100,000 inhabitants.1 Macapá, the capital city, is known for its close contact and transit with bordering countries, especially French Guyana. The introduction of HIV-1 in the Tiriyo Indian community from Amapá state has been associated with contacts with prostitutes from Suriname.36,37
HIV-1 genetic diversity in Amapá, first described in 2009, showed a clear predominance of subtype B (>95%) in PR and ENV sequences while low rate of subtype F1 was found in PR sequences.21 A subsequent molecular study from 2015 in ARV-naïve patients from Amapá reported that subtype B was the most prevalent (<70%), followed by moderate rates (<15%) of subtype F1 and BF1 recombinants and low prevalence of subtype C, CRF31_BC-like and CRF02_AG-like.26 Results and methodology of these studies21,26 are summarized in Table 1 and in the Supplementary Table. In the study from 2015, a NNRTI mutation was detected in one patient26 (Table 2). In the last HIV-BResNet survey, analyses of 10 pol sequences from Amapá state did not detect any sequence harboring SDRM7.
Remarks: The two studies including PR, RT, ENV sequences of adult patients from Amapá showed the predominance of subtype B (>70%), low to moderate rate of subtype F1, low rate of subtype Cand CR31_BC-like and CR02_AG-like strains (Supplementary Figure). The scarce molecular data on drug resistance mutations show low TDR rate26 and the absence of DRM on the national survey from 2018.7 The lack of molecular data in the important bordering state of Amapá highlights the need to monitor molecular features of HIV-1, both at level of genetic diversity and DRM in this state.
Acre stateThe Acre state (territorial area: 164,123 km2, population: 894,470 inhabitants; population density: 4.47 inhabitants/km2; HDI:0.663)2 has reported 1300 AIDS cases from 1980 to 2020.1 The AIDS detection rate in 2018 was below the national level (9.5/100,000); however from 2009 to 2019 the coefficient of AIDS related deaths increased by a 100%.1
So far, just one study from the Northern region, that included 12 sequences from patients failing ART living in Acre, described HIV-1 subtypes: highly predominant subtype B (>90%) and moderate rate of BF1 recombinants.28 The most recent HIV-BResNet survey included five sequences from Acre and none of them showed any SDRM.7 For further study details 22, 7 see Table 1, Supplementary Table and Table 2.
Remarks: All that is known about molecular features of HIV-1 in patients from Acre refers to data from 17 HIV-1 sequences collected from 2016 to 2018 in which subtypes were described in 12 samples (Supplementary Figure) and DRM was reported in five patients. This very restricted sampling, in a state which registered 100% increase in AIDS-related deaths in the last decade, shows the clear need of investments for molecular studies to better describe HIV-1 genetic diversity and the actual rates of TDR in ARV-naïve and of ADRM in ARV-experienced patients living in Acre.
Concluding remarksThe knowledge on HIV-1 genetic diversity and drug resistance mutations in patients from the seven states of the Northern region remains extremely scarce notwithstanding the public health and epidemiologic importance of this uncontrolled HIV/AIDS epidemic. Overall the lack of HIV-1 molecular data from Northern Brazil points out the great challenges to generate sound scientific data in underprivileged areas. In general, the Northern region population has been largely underrepresented in most studies, including the first two national surveys to monitor HIV-1 subtypes and transmitted drug resistance mutations in naive populations, which included a limited number of samples from Pará state only and these data were presented in conjunction with another state located outside the Northern region. Only the last HIV-BResNet survey from 2018 included samples from all the seven Northern region states and showed individual data on SDRM; however, data on HIV-1 genetic diversity by state was not disclosed.
Considering the seven states that comprise the Northern region, the two largest and most populous ones (Pará and Amazonas) have few detailed publications and regarding most of the other states, available data is based solely on a couple of studies. The Northern region is extremely vast and includes states with very peculiar geographic, ethnic and socioeconomic features, so that detailed investigations of molecular features of HIV-1 in each state are important to identify the introduction, generation and dissemination of new strains and for designing improved preventive and control measures.
The scarcity of molecular data on HIV-1 in Northern Brazil also highlights the demand and need of continued governmental support for research activities to be conducted by staff of local universities and research institutes, located away from the larger industrialized areas, as the ones in the Northern region. This reality largely reflects the pronounced asymmetry and the enormous challenges to reach more consistent capacity building for science and research in Brazil. This scenario also indicates the urgent need to train local young researchers which counting on adequate infrastructure and financial support, can act as catalyzing agents to promote technology transfer and to generate high quality, competitive scientific knowledge about the specific local public health needs of the Amazon region.
Besides the vast territorial area in a geographically isolated context, boundaries with several South American countries facilitate migration influx as the flow of Haitians after the earthquake in 2010 and more recently the flow of Venezuelans after the socioeconomic and political crisis, overwhelming the already precarious public health services and bringing potential detrimental impact not only on the management of HIV-1, but also on other infectious and chronic diseases. Recently, during the ongoing COVID-19 pandemic, the hard to reach Northern region states since the beginning have been hardly beaten by the pandemic with collapsed health infrastructure, affecting specially the most vulnerable populations.38 Despite concerns that COVID-19 may threaten the continuity of the HIV care in Brazil, the Ministry of Health claims that HIV/AIDS actions are being sustained during the pandemic.39,40 The serious threat and overburden of public health service due to the COVID-19 pandemic in Brazil, particularly in the Northern region, can thwart advances and efforts for the control of not only the HIV/AIDS epidemic but also of other infectious and chronic diseases. Therefore, efforts should be made to promote surveillance studies on the HIV/AIDS epidemic in Northern Brazil, including HIV-1 molecular features, which are important to assure better preventive and control measures.
This study was sponsored by Fundação de Amparo à Pesquisa do Estado do Amazonas (FAPEAM) grants from Pro-Estado Program, #002/2008, #007/2018, #005/2019, PAMEQ Program #004/2019 and PAPAC Program #005/2019. MMAS is PVN-II research fellow from the FAPEAM, PECTI-AM/SAÚDE Program (Grant #004/2020) and a research fellow from the Brazilian Research Council/ CNPq (“Bolsista de Produtividade em Pesquisa, Grant # 311986-2019-6).