Although the pattern of proinflammatory cytokines induced in COVID-2019 is similar to that of rheumatoid arthritis, the association of arthritis with SARS-CoV-2 infection is extremely rare and the symptoms are generally acute and self-limited. Herein we present the clinical case of a child who developed chronic arthritis after SARS-CoV-2 infection. An 11-year-old girl started with symptoms of multisystem inflammatory syndrome temporally associated with COVID-19 infection and subsequently developed chronic arthritis. After six weeks of arthritis, corticosteroids were started which resulted in clinical improvement after two weeks of use. Serology for SARS-CoV-2 was positive in the fifth week after symptom onset. Currently, the patient has no clinical complaints but continues to experience morning stiffness, high erythrocyte sedimentation rate, and synovial hypertrophy with no power Doppler signal on ultrasound. We alert to the possibility that SARS-CoV-2 may be a trigger of chronic arthritis.
The pattern of proinflammatory cytokines induced in coronavirus disease 2019 (COVID-19) shares similarities to that of rheumatoid arthritis (RA), suggesting a similar disease mechanism.1 However, the musculoskeletal symptoms attributed to infection with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) are generally acute and self-limited and arthritis is a rare symptom.2
Previous publications highlighted the important role of viral infections in the development of autoimmune diseases.3 Classic examples include type 1 diabetes, IgA vasculitis, and antiphospholipid antibody syndrome observed after an influenza outbreak,4 and transverse myelitis, arthralgia, myalgia and arthritis which are reported following Zika5 and Chikungunya.6 To our knowledge, the occurrence of chronic arthritis after SARS-CoV-2 infection has not yet been reported.
In this case report, we present the case of a girl who developed chronic arthritis after COVID-19.
Case reportAn 11-year-old previously healthy Brazilian girl attended the Emergency Department of the University Hospital of the São Paulo University, Ribeirão Preto, on July 6, 2020. The first symptom was fever (four daily peaks), which was responsive to antipyretic drugs, accompanied by prostration and non-suppurative bilateral conjunctivitis. Three days later, the child developed myalgia and arthralgias in the ankles, wrists and knees. On the fifth day of this condition, the patient started to develop a diffuse pruritic erythematous rash associated with worsening of myalgia and difficulty walking due to pain in her knees, which made her seek medical care. She denied respiratory or gastrointestinal symptoms.
The patient lives with her parents and maternal grandmother. The child's father had a cough, rhinorrhea, myalgia, and fever that had started approximately two days before the patient's symptoms. The father's coronavirus serology test collected earlier than the official recommendation was negative and reverse transcription polymerase chain reaction (RT-PCR) testing for COVID-19 was not performed. The father remained in self-quarantine.
On admission, the child had fever, tachycardia and hypertension (blood pressure at P95 + 12 mmHg percentile), but was in good general condition and showed no respiratory symptoms. She had a diffuse maculopapular rash with no scaling, sparing the face, bilateral non-suppurative conjunctival hyperemia, mild hyperemia in the tonsillar arches in the absence of enlarged lymph nodes, and painful palpation of the ankles, wrists and elbows without swelling.
An extensive infectious workup was negative (including PCR for dengue fever, Zika, Chikungunya, and coronavirus and serological tests for hepatitis A, B and C, human immunodeficiency virus, Epstein-Barr virus, cytomegalovirus, and toxoplasmosis). The electrocardiogram and echocardiogram were normal. Other complementary tests are described in Table 1.
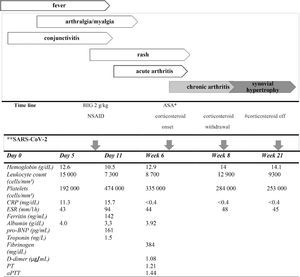
Timeline of the clinical and laboratory data of a child with chronic SARS-CoV-2-related arthritis.
*Antiaggregant dose: 5 mg/kg day; # without corticosteroid use for 4 weeks; ** epidemiological association and positive serology. HIG: human immunoglobulin; ASA: acetylsalicylic acid; NSAID: nonsteroidal anti-inflammatory drug; ESR, erythrocyte sedimentation rate; CRP, C-reactive protein; pro-BNP: pro-B-type natriuretic peptide; PT: prothrombin time; aPTT: activated partial thromboplastin time.
Due to the impossibility of performing serology at that time but with positive epidemiology for COVID-19, treatment for multisystem inflammatory syndrome in children and adolescents (MIS-C) and atypical Kawasaki syndrome temporally associated with SARS-CoV-2 infection, consisting of a single dose of human immunoglobulin (2 g/kg) was administered and an anti-inflammatory dose of acetylsalicylic acid (AAS; 1 g), every 8 hours, was initiated.7 The fever resolved within approximately 48 hours. Three days later, the child developed severe arthritis in the ankles, knees, elbows, wrists, and interphalangeal joints. The anti-inflammatory dose of AAS was maintained due to the presence of arthritis but was reduced to an anti-aggregating dose 10 days later because of elevation in hepatic transaminases.
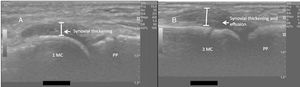
The patient initiated outpatient follow-up with the pediatric rheumatology team at the same hospital. The second echocardiogram performed 20 days after the onset of symptoms was normal. During follow up, polyarthritis in the metacarpophalangeal, interphalangeal, metatarsophalangeal, ankle and knee joints persisted, accompanied by joint ultrasound-detected synovitis with power Doppler signal (Fig. 1). The ophthalmological evaluation was normal, and antinuclear antibodies (ANA) and rheumatoid factor (RF) were negative. At five weeks after symptom onset, serology for SARS-CoV-2 (chemiluminescence) was positive, meeting the World Health Organization criteria for MIS-C.7 At six weeks of arthritis associated with a persistent increase of inflammatory markers, corticosteroids (30 mg/day) were started due to the hypothesis of persistent MIS-C inflammation. The clinical arthritis resolved within two weeks of corticosteroid use and the medication was gradually withdrawn.
Long axis (sagittal) ultrasound view of active synovitis of the first (A) and third (B) metatarsophalangeal joint in a pediatric patient with chronic arthritis retaled to SARS-CoV-2. PP: proximal phalanx; MC: Metacarpal. The images were kinfdly provided by Dr. Leonor Garbin Savarese of the Department of Medical Imaging, Oncology and Hematology, University of São Paulo, Ribeirão Preto.
At present, five months after the onset of symptoms, the child remains asymptomatic, except for complaints of morning stiffness for 30 minutes and an elevated erythrocyte sedimentation rate one month after complete corticosteroid withdrawal (Table 1). Ultrasound control detected discrete synovial hypertrophy with no power Doppler signal.
DiscussionMusculoskeletal symptoms are common in COVID-19 infection, described in 10 to 25% of patients. The main symptoms are arthralgia, fatigue, back pain, myalgia, and myopathy, including necrotizing myopathy.8–11 However, acute arthritis is a rare symptom.8
Evidence in the literature indicates a relationship between coronaviruses and arthritis, with endemic human coronavirus being associated with the development of RA.1 In a Korean study, infections with endemic human coronavirus, parainfluenza virus and metapneumovirus were associated with an increased incidence of RA.12 However, to date, there was no evidence that SARS-CoV-2 infection could induce chronic inflammatory arthritis.
Regarding the pediatric age group, clinical studies have shown that juvenile idiopathic arthritis (JIA) can occur after infectious diseases caused by viruses or bacteria, called potential triggers.13 There are several case reports with descriptions of pediatric patients who developed JIA after parvovirus B1914 and Epstein Barr virus15 infections. The association between the development of JIA and infection with Salmonella spp., Shigella spp., Campylobacter spp., Mycobacterium tuberculosis, Chlamydia trachomatis and Mycoplasma pneumoniae has also been described.16
Autoimmunity related to infectious triggers can be explained by molecular mimicry associated with increased immunogenicity after infections and polyclonal lymphocyte activation.17 The pattern of proinflammatory cytokines detected in COVID-19 is closely related to those considered key cytokines in the pathogenesis of RA. In both diseases, high levels of interleukins (IL-6, IL-1, IL-17), granulocyte-monocyte colony-stimulating factor and tumor necrosis factor are found, which cause considerable damage to the pulmonary epithelium and to the synovial membrane. In addition, T-cell activation and increased neutrophil influx contribute to alveolar and synovial injury.1 Although JIA may have some pathophysiological similarities with RA, there are no studies that correlate the possible inflammatory microenvironment shared between COVID-19 and JIA.
With respect to COVID-19 infection in pediatric patients, children were found to develop milder symptoms than adults, estimating that only 2% of cases occur in children under 19 years of age.18 Since the first case of the possible association of SARS-CoV-2 infection with inflammatory syndromes in children published in April 2020,19 several other publications reported syndromes associated with the virus, like Kawasaki disease, toxic shock syndrome, and secondary hemophagocytic lymphohistiocytosis/macrophage activation syndrome.7 Other isolated extrapulmonary manifestations such as myocarditis and neurocognitive symptoms have been described.8 The emergence of these clinical cases with epidemiological evidence of SARS-CoV-2, especially in children, has become a major concern in this age group. These manifestations were described in the clinical profile of a syndrome called “multisystem inflammatory syndrome in children” (MIS-C).7 Even in MIS-C, musculoskeletal manifestations are not part of the case definition criteria. Our patient met the MIS-C classification criteria, including the laboratory evidence of SARS-CoV-2 infection, and was treated following the current recommendations.20
ConclusionWe cannot affirm whether arthritis is part of MIS-C or a possible trigger of JIA. However, considering the descriptions of the temporal and/or causal links between viral infections and the onset of JIA, the possibility of an association between COVID-19 and chronic arthritis in the pediatric age group cannot be ruled out. Further studies are still needed to determine whether this event is a coincidence or maintains an etiopathogenic relationship with SARS-CoV-2 infection. In the case reported, we alert for the inclusion of MIS-C as a possible cause of chronic arthritis in children.
FundingNo Funding.
Informed consentThe study was approved by the Ethics Committee of the University Hospital of the Ribeirão Preto Medical School, University of Sao Paulo (Approval number 4.489.380), and the patient and her parents gave written informed consent to participate.
Declarations Ethics approval and consent to participateThe study was approved by the Ethics Committee of the University Hospital of the Ribeirão Preto Medical School, University of Sao Paulo (Approval number 4.489.380), and the patient and her parents gave written informed consent to participate.
Consent for publicationWritten informed consent was obtained from the patient's parents to publish her personal or clinical data.
Availability of data and materialsThe datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Author detailsDepartment of Pediatrics1, Division of Pediatric Rheumatology2, University Hospital of the Ribeirão Preto Medical School, University of São Paulo, Brazil.
Authors' contributionsLRMP, FHRG and LMC designed the study and wrote the paper; MMNF and MPMM were responsible for data collection and assessment. All authors read and approved the final version of the manuscript.
Not applicable.