Brazil is one of the 22 countries that concentrates 80% of global tuberculosis cases concomitantly to a large number of hepatitis C carriers and some epidemiological risk scenarios are coincident for both diseases. We analyzed tuberculosis cases that occurred during α-interferon-based therapy for hepatitis C in reference centers in Brazil between 2001 and 2012 and reviewed their medical records. Eighteen tuberculosis cases were observed in patients submitted to hepatitis C α-interferon-based therapy. All patients were human immunodeficiency virus-negative. Nine patients (50%) had extra-pulmonary tuberculosis; 15 (83%) showed significant liver fibrosis. Hepatitis C treatment was discontinued in 12 patients (67%) due to tuberculosis reactivation and six (33%) had sustained virological response. The majority of patients had a favorable outcome but one died. Considering the evidences of α-IFN interference over the containment of Mycobacterium tuberculosis, the immune impairment of cirrhotic patients, the increase of tuberculosis case reports during hepatitis C treatment with atypical and severe presentations and the negative impact on sustained virological response, we think these are strong arguments for latent tuberculosis infection screening before starting α-interferon-based therapy for any indication and even to consider IFN-free regimens against hepatitis C when a patient tests positive for latent tuberculosis infection.
Tuberculosis (Tb) is an air-borne infectious disease caused by the Mycobacterium tuberculosis, considered priority for surveillance and treatment by the World Health Organization because of its high incidence and mortality rates, especially in countries with socio-economic and sanitary issues.
Brazil is one of the 22 countries in the world that concentrate 80% of global Tb cases, ranking 16th in absolute number of cases (71,123 cases in 2013) and 22nd in incidence rate (35.4 cases/100,000 inhabitants). Most of the reported cases occur among men in the age group from 40 to 59 years, with pulmonary presentation (86%).1
Some groups are more susceptible to Tb than the general population, such as patients living with the human immunodeficiency virus/acquired immunodeficiency syndrome (HIV/Aids), patients under immunosuppressive or immunobiological therapy as with rheumatic, inflammatory bowel disease, and also in diabetis, chronic kidney disease, and solid organ transplanted patients.
The treatment of Tb with rifampicin, isoniazid and pyrazinamide was adopted in Brazil until 2009. From 2010 on, the drug regimen containing rifampicin, isoniazid, pyrazinamide, and ethambutol was adopted. Alternative therapy is recommended for patients with liver diseases or aminotransferases abnormalities, including streptomycin, ofloxacin, and ethambutol (SOE).2
The standard therapy for chronic hepatitis C has changed significantly during the years. Initially patients were treated with conventional interferon. When pegylated interferon became available patients had a more convenient and effective therapy with a duration of 24–48 weeks depending on the hepatitis C virus (HCV) genotype and fibrosis stage. In special situations such as end-stage renal disease (ESRD) isolated IFN was recommended due to the high risk of hemolytic anemia induced by ribavirin. This association of drugs could also lead to a relative immunosuppression. Nowadays several direct acting antiviral drugs (DAA) are available with high cure rates, shorter duration of treatment, and fewer adverse events. Even in this new era of CHC treatment, IFN will still be used in some situations such as genotype 3 infection and rescue therapy for non-responders.3
Hepatitis C patients treated with α-interferon (α-IFN) and ribavirin are not yet characterized as a more susceptible group for developing Tb, although it is already known that there is an increase on hepatitis C prevalence among Tb patients when compared to the general population.4 Indeed, some epidemiological risk scenarios are coincident for both diseases, such as incarceration, homelessness, and drug addiction.1 Other risk factors for Tb such as undernourishment and relative immunodepression can also be induced by α-IFN-based hepatitis C treatment.5
To the extent of our knowledge, at least 16 Tb cases during or soon after interferon-based hepatitis C therapy in non-HIV patients were reported in 15 articles so far, some of them describing severe outcomes of tuberculosis reactivation.6–15
The aim of the present study was to analyze 18 cases of Tb observed in reference centers for hepatitis C treatment in Brazil, country with a high prevalence of Tb and an expressive number of hepatitis C carriers (2–3 million people).3
We retrospectively analyzed the records of all treated patients for hepatitis C which were seen monthly during therapy and identified, based on the signs and symptoms, the occurrence of Tb cases under various clinical presentations in patients submitted to α-IFN-based therapy for hepatitis C (pegylated or not).
Patients were evaluated between 2001 and 2012, in four reference centers in Brazil (two in Sao Paulo, one in Rio de Janeiro and one in Maranhao where the peak of incidence of tuberculosis during this period was 43.7/100,000 in 2001, 93.9/100,000 in 2001 and 47/100,000 in 2002, respectively).16 Cases were considered related to hepatitis C treatment when diagnosed during or within six months after the end of treatment.
Once the cases had been identified, their medical records were reviewed to obtain epidemiological, clinical, laboratory, and therapeutic characteristics related to hepatitis C and Tb and the data about the decision to interrupt treatment. This decision was made by clinical judgment based on the severity of the disease.
This study reports secondary data from a main study approved by the Ethics Committee for Clinical Research of the main study center.
We identified 18 cases of Tb in our study, with an incidence rate of 809 cases/100,000 treated patients.
The majority of patients were male (78%), with a mean age of 49±10 years; 15 patients (83%) had significant liver fibrosis, including six (33%) with cirrhosis; HCV genotype 1 was the most frequent (78%). The mean time under hepatitis C treatment until the onset of Tb symptoms was 33±15 weeks. The mean hemoglobin level on Tb diagnosis was 12.1±4.2g/dL and the mean of neutrophil counts was 3596±1938cells/mm3.
Most patients were treated for hepatitis C with double therapy, including α-IFN (pegylated or not) associated with ribavirin (89%). The treatment was discontinued early in 67% of cases because of Tb onset. In an intention to treat approach, only six patients (33%) achieved sustained virological response (SVR), while five relapsed (RR), and the remaining were non-responders (NR).
At least nine patients (50%) had other risk factors for Tb development (diabetes mellitus, chronic kidney disease, tobacco smoking, or alcohol intake). Both Diabetes mellitus and chronic kidney disease coexisted in 17% of Tb cases. Cirrhosis was present in 33% of cases. The most frequent clinical presentation was pulmonary Tb, in 50% of cases, with half of patients developing extra-pulmonary Tb. Only one of cirrhotic patients had extra-pulmonary Tb (pleural effusion) concomitantly to pulmonary disease.
Symptoms and signs of Tb usually appeared during the third and fourth trimesters of treatment (83% of cases). Three patients presented Tb after concluding α-IFN therapy (two after three and one after six months of the end of treatment).
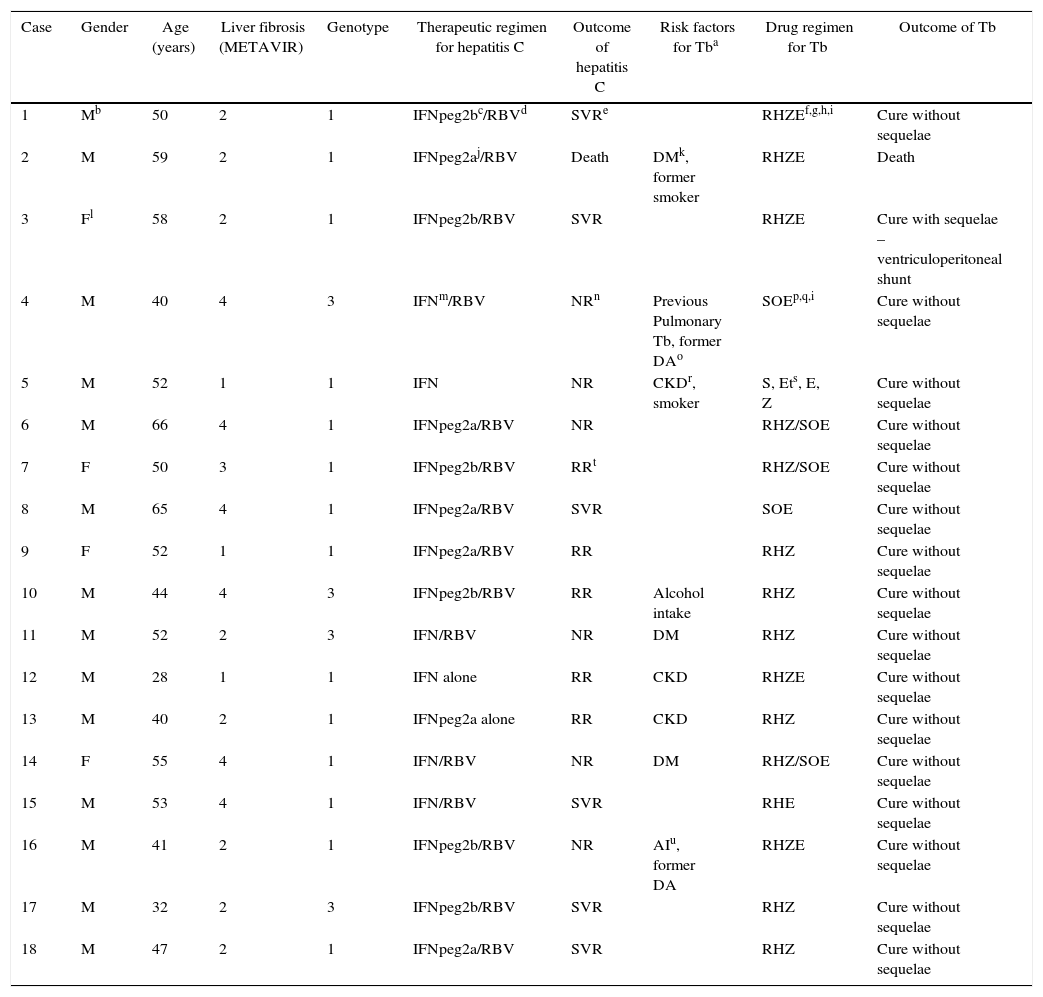
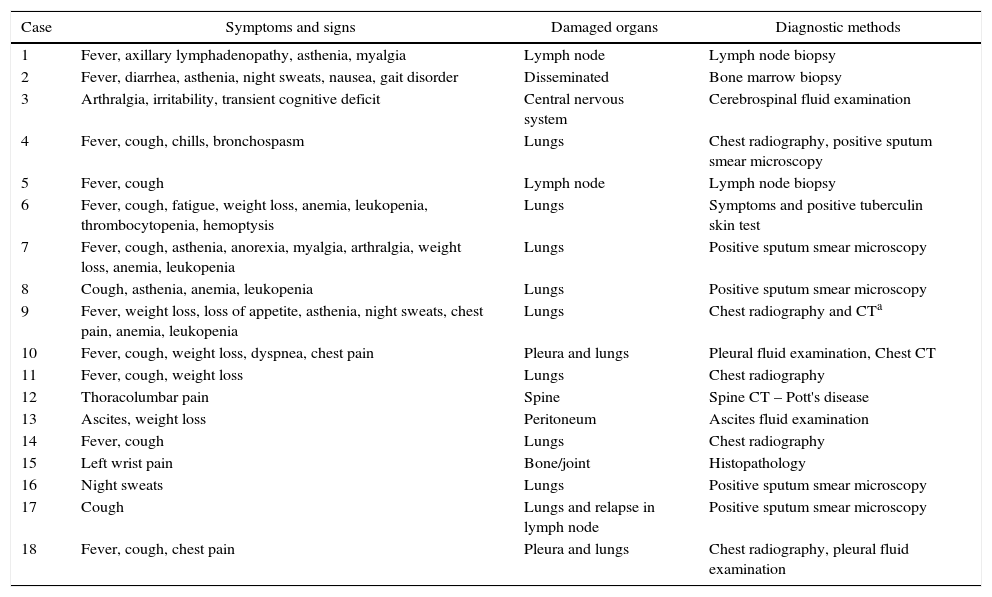
Details of treatment for hepatitis C and Tb are shown in Table 1 and clinical presentation of Tb in Table 2. Nine patients were treated with the association of rifampicin, isoniazid and pyrazinamide, adopted in Brazil until 2009 for Tb treatment. From 2010 on, the drug regimen containing rifampicin, isoniazid, pyrazinamide, and ethambutol was adopted, what explains its predominance in the four cases identified between 2010 and 2012.
Clinical/epidemiological characteristics of patients who developed tuberculosis during or soon after hepatitis C treatment.
| Case | Gender | Age (years) | Liver fibrosis (METAVIR) | Genotype | Therapeutic regimen for hepatitis C | Outcome of hepatitis C | Risk factors for Tba | Drug regimen for Tb | Outcome of Tb |
|---|---|---|---|---|---|---|---|---|---|
| 1 | Mb | 50 | 2 | 1 | IFNpeg2bc/RBVd | SVRe | RHZEf,g,h,i | Cure without sequelae | |
| 2 | M | 59 | 2 | 1 | IFNpeg2aj/RBV | Death | DMk, former smoker | RHZE | Death |
| 3 | Fl | 58 | 2 | 1 | IFNpeg2b/RBV | SVR | RHZE | Cure with sequelae – ventriculoperitoneal shunt | |
| 4 | M | 40 | 4 | 3 | IFNm/RBV | NRn | Previous Pulmonary Tb, former DAo | SOEp,q,i | Cure without sequelae |
| 5 | M | 52 | 1 | 1 | IFN | NR | CKDr, smoker | S, Ets, E, Z | Cure without sequelae |
| 6 | M | 66 | 4 | 1 | IFNpeg2a/RBV | NR | RHZ/SOE | Cure without sequelae | |
| 7 | F | 50 | 3 | 1 | IFNpeg2b/RBV | RRt | RHZ/SOE | Cure without sequelae | |
| 8 | M | 65 | 4 | 1 | IFNpeg2a/RBV | SVR | SOE | Cure without sequelae | |
| 9 | F | 52 | 1 | 1 | IFNpeg2a/RBV | RR | RHZ | Cure without sequelae | |
| 10 | M | 44 | 4 | 3 | IFNpeg2b/RBV | RR | Alcohol intake | RHZ | Cure without sequelae |
| 11 | M | 52 | 2 | 3 | IFN/RBV | NR | DM | RHZ | Cure without sequelae |
| 12 | M | 28 | 1 | 1 | IFN alone | RR | CKD | RHZE | Cure without sequelae |
| 13 | M | 40 | 2 | 1 | IFNpeg2a alone | RR | CKD | RHZ | Cure without sequelae |
| 14 | F | 55 | 4 | 1 | IFN/RBV | NR | DM | RHZ/SOE | Cure without sequelae |
| 15 | M | 53 | 4 | 1 | IFN/RBV | SVR | RHE | Cure without sequelae | |
| 16 | M | 41 | 2 | 1 | IFNpeg2b/RBV | NR | AIu, former DA | RHZE | Cure without sequelae |
| 17 | M | 32 | 2 | 3 | IFNpeg2b/RBV | SVR | RHZ | Cure without sequelae | |
| 18 | M | 47 | 2 | 1 | IFNpeg2a/RBV | SVR | RHZ | Cure without sequelae |
Clinical aspects and diagnostic methods of tuberculosis cases.
| Case | Symptoms and signs | Damaged organs | Diagnostic methods |
|---|---|---|---|
| 1 | Fever, axillary lymphadenopathy, asthenia, myalgia | Lymph node | Lymph node biopsy |
| 2 | Fever, diarrhea, asthenia, night sweats, nausea, gait disorder | Disseminated | Bone marrow biopsy |
| 3 | Arthralgia, irritability, transient cognitive deficit | Central nervous system | Cerebrospinal fluid examination |
| 4 | Fever, cough, chills, bronchospasm | Lungs | Chest radiography, positive sputum smear microscopy |
| 5 | Fever, cough | Lymph node | Lymph node biopsy |
| 6 | Fever, cough, fatigue, weight loss, anemia, leukopenia, thrombocytopenia, hemoptysis | Lungs | Symptoms and positive tuberculin skin test |
| 7 | Fever, cough, asthenia, anorexia, myalgia, arthralgia, weight loss, anemia, leukopenia | Lungs | Positive sputum smear microscopy |
| 8 | Cough, asthenia, anemia, leukopenia | Lungs | Positive sputum smear microscopy |
| 9 | Fever, weight loss, loss of appetite, asthenia, night sweats, chest pain, anemia, leukopenia | Lungs | Chest radiography and CTa |
| 10 | Fever, cough, weight loss, dyspnea, chest pain | Pleura and lungs | Pleural fluid examination, Chest CT |
| 11 | Fever, cough, weight loss | Lungs | Chest radiography |
| 12 | Thoracolumbar pain | Spine | Spine CT – Pott's disease |
| 13 | Ascites, weight loss | Peritoneum | Ascites fluid examination |
| 14 | Fever, cough | Lungs | Chest radiography |
| 15 | Left wrist pain | Bone/joint | Histopathology |
| 16 | Night sweats | Lungs | Positive sputum smear microscopy |
| 17 | Cough | Lungs and relapse in lymph node | Positive sputum smear microscopy |
| 18 | Fever, cough, chest pain | Pleura and lungs | Chest radiography, pleural fluid examination |
The 18 Tb cases reported in this study rise the possibility of a higher risk for infection in patients under α-IFN-based therapy for hepatitis C.
In a population-based study conducted in Taiwan, an endemic Tb area, Lin et al., 2014 studied the incidence rates of active tuberculosis in HCV-infected patients with (n=621) or without (n=2460) interferon-based therapy (IBT). They observed a non statistically significant increased hazard of active Tb in HCV infected patients on IBT in one-year follow-up.17 Moreover, they mention that the investigators had no access to other important Tb risk factors, detailed laboratory results, and medication records. They also consider that some patients could have died before being registered in the Health Insurance Research Database due to Tb infection or taking medications for that condition, underestimating the number of cases. The magnitude of 809 cases/100,000 treated patients is more impressive when the data are compared to the incidence of Tb cases in the Brazilian population in 2013 (35.4 cases/100,000 inhabitants). Other groups, traditionally a priority for Tb control, such as the indigenous people, have a lower incidence (94.5 cases/100,000 inhabitants) when compared to our report.1 On the other hand, comparable incidence rates had been described in renal transplant patients: 803 cases/100,000 patients.18
Most patients were male within the age group of higher prevalence of Tb in Brazil.1Diabetes mellitus and chronic kidney disease coexisted in less than 20% of cases, comorbidities widely known to increase the risk of active tuberculosis.19 Cirrhosis, also blamed as a risk factor for Tb,20 was present in one third of cases. Half of the cases were extra-pulmonary tuberculosis, more compatible with a situation of immunosuppression, since in non-immunosuppressed subjects only 15–20% of extra-pulmonary forms are expected.21
We observed a high discontinuation rate of IBT due to the onset of Tb; one third of patients achieved SVR. On case two, the acute onset of Tb symptoms and severe and fatal outcome of the disease in less than 60 days, bring the possibility of bacteremia and sepsis caused by M. tuberculosis in an early phase of infection.15
On the previous case reports the authors discussed many mechanisms of association between hepatitis C treatment and Tb reactivation. They suggested that IFN can induce an impairment of the early immune response against M. tuberculosis, specifically observed on severe cases, besides the role of leukopenia, neutropenia, a lowering on T-CD4+ lymphocyte population and abnormalities on chemotaxis and phagocytic functions of macrophages5,8,10,11,15.
We would like to emphasize that many studies had already showed that α- and β-IFN inhibit type I immune response which is characterized by lL-12, γ-IFN and TNF-α production, cytokines that restrain M. tuberculosis. This inhibition could lead to LTBI reactivation,22 as occurred on most cases reported here, including the three cases in which Tb appeared after stopping hepatitis C treatment. We consider that there was a residual immune effect of α-IFN that can last until 16 weeks after the last dose administered.
In summary, considering the evidences of α-IFN interference over the constrainment mechanisms of M. tuberculosis, the immune impairment of cirrhotic patients, the increase of Tb case reports during hepatitis C treatment, as in this expressive retrospective study, with atypical and severe presentations, and the negative impact on SVR, we think that these are strong arguments for LTBI screening by tuberculin skin test and/or by interferon gamma releasing assays, before starting α-IFN-based therapy. This is even more important in countries with high Tb incidence and in more susceptible populations in which epidemiological determinants of both diseases can coexist and interact. In these medium or low income countries the use of α-IFN-based therapy will still persist for some time before it is possible to extend the use of direct-acting antivirals (DAA) for hepatitis C. DAA should be considered if a patient tests positive for LTBI. Furthermore, even in the direct active antivirals era, the inclusion of patients in the treatment of hepatitis C is an opportunity for LTBI screening.
Conflicts of interestThe authors declare no conflicts of interest.