Hepatitis B virus (HBV) infection is a major public health problem in Brazil. HBV endemicity is usually moderate to low according to geographic regions, and high prevalence of this virus has been reported in people of some specific Brazilian counties, including those with a strong influence of Italian colonization in southern Brazil. Analysis of HBV diversity and identification of the main risk factors to HBV infection are necessary to understand hepatitis B epidemiology in these high prevalence regions in southern Brazil.
ObjectiveTo investigate epidemiological characteristics and HBV genotypes and subgenotypes circulating in a specific city with high HBV prevalence.
MethodsA cross-sectional study was performed with 102 HBV chronically infected individuals, recruited in reference outpatient clinics for viral hepatitis in a city of high HBV prevalence (Bento Gonçalves) in Rio Grande do Sul state, Brazil between July and December 2010. Socio-demographic, clinical and behavior-related variables were collected in a structured questionnaire. HBV serological markers (HBsAg, anti-HBc), viral load, genotypes/subgenotypes and drug resistance were evaluated and comparatively analyzed among all patients.
ResultsThe HBV infected subjects had a mean age of 44.9 (±12.2) years, with 86 patients (84.3%) reporting to have a family history of HBV infection, 51 (50.0%) to share personal objects, and were predominantly of Italian descendants (61; 64.9%). There was a predominance of genotype D (49/54; 90.7%), but genotype A was also detected (5/54; 9.3%). Subgenotypes D1 (1; 4.7%), D2 (3; 14.3%), and D3 (17; 81.0%) were identified. LAM-resistant mutation (rtM204I) and ADV-resistant mutations (rtA181V) were detected in only one patient each.
ConclusionsThese results demonstrate a pivotal role of intrafamilial transmission for HBV spreading in this population. Furthermore, there is a high prevalence of HBV genotype D in this region.
Hepatitis B virus (HBV) infection is one of the most important human diseases with about two billion people infected worldwide, including 240–280 million with chronic hepatitis B.1 HBV-infected individuals can develop cirrhosis, hepatocellular carcinoma and other hepatic injuries.2 More than 600,000 people die each year due to clinical hepatic complications by HBV infection.3
Taxonomically, HBV belongs to the Hepadnaviridae family and presents a partial double strand DNA genome of approximately 3200 base pairs.4 Ten HBV genotypes (A to J) have already been described, with nucleotide sequences divergences greater than 8% in the entire viral genome. In Brazil, the most frequent genotypes are A (58.7%), D (23.4%), and F (11.3%), with A more frequent in Southeast, North and Northeast regions, while D predominates in the South region.5 Most genotypes present subgenotypes that may lead to different diagnostic and clinical profiles.4
HBV transmission occurs via contact with infected blood and body fluids (WHO 2016). Infection via contacts with medical/dental devices,6,7 piercings use,8 tattoo,9 and illicit drugs use (principally injected) have been reported.10 Further, this transmission may also occur in the family context11,12 and sharing of personal objects with family members is strongly associated with HBV transmission.13 In this sense, recent studies have reported important associations between HBV transmission in intrafamilial environmental.13–15
HBV vaccine has been used in Brazil in the last 20 years.16 Immunization programs started in the infant population in the 1990s and gradually covered the rest of the population. Vaccination coverage for HBV reaches approximately 50% of the population and the effectiveness is estimated at 60%.10,16 Despite elderly patients have higher rates of HBV infection (most of them were infected before the availability of the vaccine), contamination has been demonstrated in all age groups.16
HBV therapy is also largely used and aims to prevent progression to more severe clinical conditions as cirrhosis and hepatocellular carcinoma. The most used drugs in the last years were immunomodulators, such as interferon-alpha and pegylated interferon alpha, and nucleos(t)ide analogues, such as lamivudine (LAM), adefovir (ADV), entecavir (ETV), telbivudine (LdT) and tenofovir (TDF).17 LAM was the main antiviral drug used in the HBV treatment, but this drug was associated with high rates of drug resistance-related mutations in the HBV polymerase.18 Currently, the treatment of choice is TDF (disoproxil or alafenamide) since it has a high genetic barrier for drug resistance. The combination of therapies is not commonly recommended.17
Laboratory diagnosis is usually based on serology. Previous studies demonstrated markers for HBV infection in 12.7% (anti-HBc positive) and 0.7% (HBsAg positive) of the Brazilian population.16 The highest HBV detection rate in the country (17.2 cases per 100,000 inhabitants) was observed in South Region, but even there the HBV prevalence was low. In this same geographic region, HBV frequency also increases with age: 1.6% anti-HBc positive in the range of 10–19 years old versus 11.3% among those aged 20–69 years.10 In this whole region, some areas have been more studied because of the high HBV prevalence. It is noteworthy that these areas are generally of Italian descendants and with a high prevalence of genotype D.19–21
The present report is a cross-sectional study aimed to assess epidemiological risk factors for HBV infection, as well as to determine HBV genotypes/subgenotypes and resistance mutations in a sample from Bento Gonçalves, a city with a high prevalence of this disease in southern Brazil.
MethodsSubjects and data collectPatients over 18 years old were recruited between July and December 2010 from two reference outpatient clinics for hepatitis in the city of Bento Gonçalves, Rio Grande do Sul state. The inclusion criterion was diagnosis of “chronic hepatitis B” (detectable HBsAg for more than six months in two tests performed before the beginning of the present study). Informed consent was obtained from all patients previous to the inclusion in the study. The study meets all applicable ethical standards for experimentation and research integrity, according to the Declaration of Helsinki.
Socio-demographic and potential risk factors for HBV infection were obtained through a standardized individual questionnaire that was administered by a trained interviewer in a private room. The Alcohol Use Disorders Identification Test (AUDIT), validated in Brazilian Portuguese, was used to screen for alcohol use disorders. A score ≥ 8 was considered alcohol abuse.22 General clinical and laboratory data, including alanine aminotransferase (ALT) and aspartate aminotransferase (AST), creatinine, albumin, prothrombin time (PT), ascites, and liver biopsies were obtained from medical records, using data from the most recent tests at the time of interview. Liver biopsies were classified according to the METAVIR scoring system (fibrosis stage on a five-point scale, with F0 = no fibrosis, F1 = portal fibrosis and periportal without septations, F2 = portal and periportal fibrosis with rare septations, F3 = portal fibrosis and periportal with many septations, F4 = cirrhosis; necroinflammatory activity estimating the degree of portal inflammatory lesions and hepatocellular necrosis on a four-point scale, A0 = no activity, A1 = mild, A2 = moderate, A3 = severe). The study was approved by the Ethics Committees of Hospital de Clínicas (Porto Alegre, RS, Brazil), Universidade Luterana do Brasil (Canoas, RS, Brazil) and Hospital Tacchini (Bento Gonçalves, RS, Brazil).
Sampling and laboratory testsBlood samples (12 mL) were collected in the same moment for all the laboratory analysis. Serological tests were performed by commercially available assays: HBsAg using ETI-MAK-4 (DiaSorin, Saluggia, Italy) and anti-HBc using ETI-AB-COREK PLUS assay (DiaSorin, Sallugia, Italy).
Total DNA was extracted according to previously described methodology.23 Real-time polymerase chain (PCR) reaction was used for HBV detection and quantification, as previously described.24 Both negative and positive controls were included in each PCR run. HBV-DNA positive samples were further submitted to genotyping by amplification and sequencing of a 359 bp fragment of the reverse transcriptase (P gene). The primers pairs used for this PCR were: 5′-CAATGTGGWTAYCCTGCYTTAATGCC-3′ and 5′-GCACAGCCTAGCWGCCA TGG-3′. The PCR conditions consisted of 40 cycles of 94 °C for 15 s, 60 °C for 30 s, 72 °C for 120 s.
Twenty-one HBV positive samples presenting viral load higher than 2000 IU/mL were further submitted to sequencing of a larger fragment of 590 bp encompassing a region of the reverse transcriptase (P gene) and the overlapping S gene (surface antigen HBsAg gene). This region was selected for analysis of resistance to antivirals and was amplified by nested PCR. The primers pairs used were 5′-CASTCATCCWCAGGCMATGCAGTGGA-3′ and 5′-GGGTTGCGTCAGCAAACACTTGGC-3′ in the first round; and 5′-CATCCTGCTGCTATGCCTCATCTTC-3′ and 5′-ATDCKTTGACADACTTTCCARTCAAT-3′ in the second round of amplification. The first-round PCR consisted of 25 cycles of 94 °C for 15 s, 55 °C for 30 s, 72 °C for 120 s. The second-round PCR consisted of 35 cycles of 94 °C for 15 s, 60 °C for 30 s, 72 °C for 120 s.
Nucleotide sequences were obtained by Data Collection v1.0.1 (Applied Biosystems) and electropherograms were analyzed with Sequencing Analysis v.5.3.1. software (Applied Biosystems) and edited in SeqMan (DNAStar, Madison, WI, USA). Nucleotide and amino acids sequences were aligned by the MAFFT method25 and phylogenies were assembled by Geneious 9.1.2 (Geneious, Inc) software. Neighbor-joining method26 was used to reconstruct phylogenetic trees, which were performed by comparing sequences obtained in this study with Genbank data representative of other genotypes and subgenotypes (NCBI Genbank: https://www.ncbi.nlm.nih.gov/genbank/). Genetic distances were calculated using General Time Reversible (GTR + I + G) nucleotide substitution model, estimated using jModeltest v.2.1.4.27 Bootstrap analysis with 1000 replicates were performed to test the reliability of the tree with values ≥ 60 indicated on the branches.
In the resistance analysis, rtM204I/V was defined as the signature of LAM-resistant mutations (LAM-R) and also encompassing resistance to LdT (LdT-R), rtA181V and rtN236T were defined as the signature of ADV-resistant mutations (ADV-R) and rtT184A/C/F/G/I/L/M/S, rtS202C/G/I and rtM250I/L/V were defined as the signature ETV-resistant mutations (ETV-R).28
Statistical analysisData were analyzed using the Statistical Package for Social Sciences (SPSS, version 18.0, Chicago, IL). The distributions of the quantitative variables were evaluated by the Kolmogorov–Smirnov test with Lilliefors correction. Variables with normal distribution were compared by Student’s t-test and those with non-parametric distribution were compared by Mann–Whitney test. Categorical variables were compared by Chi-Square or Fisher’s Exact Test, according to the recommendation. All statistical tests in this study were two-sided and p-values < 0.05 were considered as statistically significant.
ResultsThe epidemiologic profile of HBV patientsOne hundred and two HBV chronically infected patients were included in the study. The mean age was 44.9 ± 12.2 years and there were 55 males (53.9%) and 47 females (46.1%). No significant differences between sexes were observed in most socio-demographic variables, excepting higher level of schooling and less than five siblings (Table 1). Most patients were married (n = 79, 77.5%), lived in rural area of the municipality (n = 68, 66.7%) during childhood, self-defined white ethnic group (n = 76, 74.5%), and were predominantly of Italian descendants (n = 61, 64.9%) (Table 1). There was a great heterogeneity related to occupation of the individuals, including the economic segments of industry/commerce (n = 82, 80.4%), farming (n = 8, 7.8%), and housewife/housemaid (n = 6, 5.9%).
Socio-demographic characteristics of HBV patients stratified by sex (Bento Gonçalves, RS, Brazil, 2010).
| Variables | Total (n = 102) | Men (n = 55) | Women (n = 47) | p-Valuea |
|---|---|---|---|---|
| n (%) | n (%) | n (%) | ||
| Age (mean ± SD) | 44.9 ± 12.2 | 46.3 ± 12.8 | 43.3 ± 11.3 | 0.22 |
| Level of schooling | 0.01 | |||
| Elementary school or less | 55 (53.9) | 38 (69.1) | 17 (36.2) | |
| High school | 47 (46.1) | 17 (30.9) | 30 (63.8) | |
| Marital status | 0.30 | |||
| Married | 79 (77.5) | 44 (80.0) | 35 (74.5) | |
| Not married | 23 (22.5) | 11 (20.0) | 12 (25.5) | |
| Place of residence in childhood | 0.48 | |||
| Rural area | 68 (66.7) | 35 (63.6) | 33 (70.2) | |
| Urban | 34 (33.3) | 20 (36.4) | 14 (29.8) | |
| Number of siblingsb | 0.01 | |||
| >5 | 46 (45.1) | 31 (56.3) | 15 (31.9) | |
| ≤5 | 56 (54.9) | 24 (43.7) | 32 (68.1) | |
| Ethnic groupb | 0.16 | |||
| Not white | 15 (14.7) | 10 (18.2) | 4 (8.5) | |
| White | 76 (74.5) | 39 (70.9) | 37 (78.7) | |
| Occupation | – | |||
| Farmer | 8 (7.8) | 8 (14.5) | 0 (0.0) | |
| Industry/commerce | 82 (80.5) | 44 (80.0) | 38 (80.9) | |
| Housewife/housemaid | 6 (5.9) | 0 (0.0) | 6 (12.8) | |
| Retired | 1 (0.9) | 0 (0.0) | 1 (2.1) | |
| Other | 5 (4.9) | 3 (5.5) | 2 (4.3) | |
| Ancestry | ||||
| Number of grandparents Italiansb | ||||
| Four | 61 (64.9) | 32 (58.2) | 29 (61.7) | 0.63 |
| Tree | 8 (8.5) | 5 (9.1) | 3 (4.5) | |
| Two | 10 (10.6) | 2 (3.6) | 8 (17.0) | |
| One | 2 (2.1) | 1 (1.8) | 1 (2.1) | |
| None | 13 (13.8) | 10 (18.2) | 3 (6.4) |
Additionally, 90.2% (n = 92) of patients reported normal delivery, 50.0% (n = 51) shared personal objects, 49.0% (n = 50) previous use of glass syringe, 21.6% (n = 22) blood transfusion, 15.7% (n = 16) sexually transmitted infection (STI), 7.8% (n = 8) tattoo, and 4.9% (n = 5) body piercing. HBV patients reported different means of infection, such as exposure to sharp objects (n = 20, 19.6%), sexual intercourse (n = 16, 15.7%), vertical transmission (n = 16, 15.7%), blood transfusion (n = 11, 10.8%), and syringe sharing (n = 8, 7.8%) (Table 2). The family history of HBV infection was also analyzed: 86 (84.3%) patients referred having HBV-positive family members, with 42 (41.2%) patients reporting a history of HBV infection in siblings, 28 (27.5%) in the mother, and 11 (10.8%) in the father.
Risk factors for HBV infection stratified by sex (Bento Gonçalves, RS, Brazil, 2010).
| Variables | Total (n = 102) | Men (n = 55) | Women (n = 47) | p-Valuea |
|---|---|---|---|---|
| n (%) | n (%) | n (%) | ||
| History of siblings infected (HBV) | 42 (41.2) | 20 (36.4) | 22 (46.8) | 0.28 |
| History of mother infected (HBV) | 28 (27.5) | 15 (27.3) | 13 (27.7) | 0.70 |
| History of father infected (HBV) | 11 (10.8) | 7 (12.7) | 4 (8.5) | 0.52 |
| Type of deliveryb | 0.06 | |||
| Normal | 92 (90.2) | 49 (89.1) | 43 (91.5) | |
| Cesarean | 4 (3.9) | 0 (0) | 4 (8.5) | |
| Previous STI | 16 (15.7) | 11 (20.0) | 5 (10.6) | 0.18 |
| Sharing of personal objects | 51 (50.0) | 18 (32.7) | 33 (70.2) | <0.01 |
| Blood Transfusion history | 22 (21.6) | 15 (27.3) | 7 (14.9) | 0.13 |
| Tattoo | 8 (7.8) | 2 (3.6) | 6 (12.8) | 0.14 |
| Body piercing | 5 (4.9) | 0 (0) | 5 (10.6) | 0.02 |
| Previous use of glass syringe | 50 (49.0) | 28 (50.9) | 22 (46.8) | 0.58 |
| Possible forms of infection | 0.88 | |||
| Sexual intercourse | 16 (15.7) | 8 (14.5) | 8 (17.0) | |
| Blood transfusion | 11 (10.8) | 7 (12.7) | 4 (8.5) | |
| Sharp objects | 20 (19.6) | 9 (16.4) | 11 (23.4) | |
| Syringe sharing | 8 (7.8) | 4 (7.3) | 4 (8.5) | |
| Birth (vertical transmission) | 16 (15.7) | 9 (16.4) | 7 (14.9) | |
| Other forms | 30 (28.0) | 13 (23.6) | 17 (36.2) | |
| AUDIT Score (Mean ± SD) | 2.5 ± 4.3 | 3.4 ± 4.3 | 1.40 ± 4.1 | <0.01 |
| Alcohol use disorder | <0.01 | |||
| Yes | 14 (13.7) | 12 (21.8) | 2 (4.3) | |
| Smoked drugs use | 0.29 | |||
| Yes | 7 (6.9) | 5 (9.1) | 2 (4.3) | |
| Sniffed drugs use | 0.30 | |||
| Yes | 2 (1.9) | 2 (3.6) | 0 (0) | |
| Injected drugs use | 0.54 | |||
| Yes | 1 (0.9) | 1 (1.8) | 0 (0) |
STI, sexually transmitted disease; AUDIT, Alcohol Use Disorders Identification Test.
The frequencies of alcohol and illicit drugs use were also evaluated (Table 2). Men presented higher scores in the AUDIT test (3.4 ± 4.3) than women (1.40 ± 4.1), with a consequent higher frequency of alcohol use disorder (p < 0.01). Use of illicit drugs was also reported (6.9% for smoked drugs, 1.9% for sniffed, and 0.9% for injected), but they did not present any significant difference between sexes (all with p-values > 0.05) (Table 2).
HBV detection, quantitation and genotypingHBV-DNA was detected in 54 patients (52.9%) who presented a viral load mean of 3.0 ± 1.7 log10 IU/mL. Thirty-three patients had low viral load (<2000 IU/mL), including six in active treatment against HBV (four using LAM, two TDF plus LAM). Among the remaining 21 patients with a viral load > 2000 IU/mL, six were also on antiviral treatment against HBV (three using α-interferon, three LAM). Thirteen of these 21 patients underwent liver biopsy and the results demonstrated that seven (53%) did present no fibrosis, five had mild fibrosis, and only one presented cirrhosis. Necroinflammatory activity (indicating hepatocellular necrosis) was mild in seven, moderate in four, and severe in two patients (Table 3).
Clinical characteristics of HBV patients stratified by sex (Bento Gonçalves, RS, Brazil, 2010).
| Variables | Total | Men (n = 55) | Women (n = 47) | p-Valuea |
|---|---|---|---|---|
| n (%) | n (%) | n (%) | ||
| PCR | 0.45 | |||
| Positive | 54 (52.9) | 31 (56.4) | 23 (48.9) | |
| Negative | 48 (47.1) | 24 (43.6) | 24 (51.1) | |
| Diagnostic time in years (mean ± SD) | 6.5 ± 5.8 | 6.8 ± 6.1 | 6.0 ± 5.5 | 0.56 |
| HBV genotypes | 0.28 | |||
| A | 5 (9.3) | 4 (12.9) | 1 (4.3) | |
| D | 49 (90.7) | 27 (87.1) | 22 (95.7) | |
| Viral load (mean log10 IU/mL ± SD) | 3.0 ± 1.7 | 3.0 ± 2.0 | 3.0 ± 2.0 | 0.97 |
| HBV treatment | 25 (24.5) | 16 (29.1) | 9 (19.1) | 0.28 |
PCR, polymerase chain reaction.
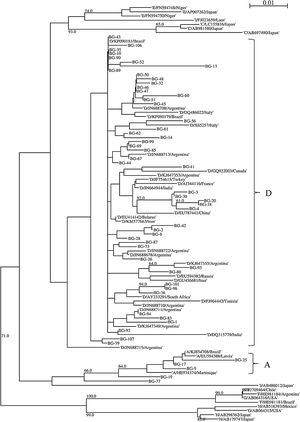
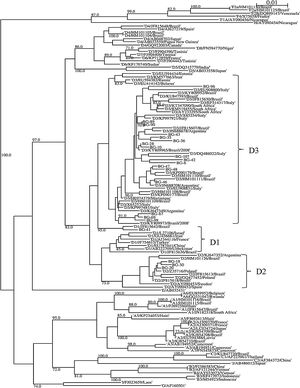
A partial region of the HBV polymerase gene (in a region coding for the reverse transcriptase) of 359 bp was sequenced in all the 54 patients. The phylogenetic analysis demonstrated that 49 (90.7%) patients were infected with genotype D and five (9.4%) with genotype A (Fig. 1). An additional HBV genotyping analysis was carried out sequencing a larger fragment of the 590 bp encompassing P/S gene in the 21 HBV-infected patients with a viral load > 2000 IU/mL (all infected with genotype D). The phylogenetic analysis demonstrated that subgenotype D3 was the most frequent (n = 17, 81.0%), but subgenotypes D2 (n = 3, 14.3%) and D1 (n = 1, 4.7%) were also detected (Fig. 2). Of these patients, 13 (61.9%) were male. In addition, Italian ancestry was predominant, of which 13 (61.9%) had four grandparents of Italian origin (Table S1).
Phylogenetic tree of P gene fragment (359 bp) of 54 patients with hepatitis B infection in Bento Gonçalves city, Rio Grande do Sul State, 2010. The sequences evaluated in this study are represented by the acronym BG (Bento Gonçalves) followed by the identification number of the sample. Sequences obtained on GenBank are demonstrated by the genotype information followed by the sequence accession number. The numbers at each node correspond to bootstrap values (greater than 60%) obtained with 1000 replicates. The scale bar indicates the genetic distances.
Phylogenetic tree of S/P gene fragment (590 bp) of 21 patients with active hepatitis B infection in Bento Gonçalves city, Rio Grande do Sul State, 2010. The sequences evaluated in this study are represented by the acronym BG (Bento Gonçalves) followed by the identification number of the sample. Sequences obtained on GenBank are demonstrated by the subgenotype information followed by the sequence accession number. The numbers at each node correspond to bootstrap values (greater than 60%) obtained with 1000 replicates. The scale bar indicates the genetic distances.
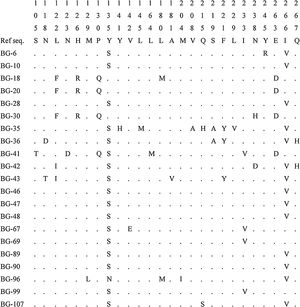
Amino acid substitutions in the P/S gene were evaluated in the 21 patients with a viral load > 2000 IU/mL (Fig. 3). A total of 26 amino acid positions presented modifications in comparison to a reference sequence (NCBI GenBank accession: X69798). The majority of the patients presented the following modifications: Y135S in 17 (80.9%) patients and I266V in 14 (66.7%) patients. Interestingly, three of the four patients without any of these modifications presented four other specific polymorphisms: L122F, H126R, P130Q and E263D. LAM-resistant mutation rtM204I was observed in only one patient (BG-96), who was on LAM treatment. This patient also presented the compensatory mutation rtL180M. The rtA181V, defined as the signature of ADV-resistant mutations, was also observed in one patient (BG-43). This patient had already been treated with lamivudine and adefovir.
DiscussionHBV infection is a serious health problem in Brazil. Recent data demonstrated a prevalence rate higher than the average in some cities and regions in South Brazil.19,21 Bento Gonçalves is one of these cities, with an incidence of 41.5 cases per 100,000 inhabitants.29 The whole city is geographically located in the mountains of the Rio Grande do Sul state, a region colonized by European immigrants, mainly Italians. Therefore, socio-demographic characteristics and risk factors were evaluated to identify the frequency of epidemiological characteristics associated with HBV infection in this population.
In this study, the socio-demographic characteristics of HBV infected patients were having elementary school or less (53.9%), be married (77.5%), living in rural area (66.7%), having ≥ 5 siblings (45.1%), of white skin color (74.5%), and of Italian ancestry (four grandparents) (64.9%). Further, considering risk factors, the patients evaluated had 41.2% of siblings, 27.5% of mothers, and 10.8% of fathers HBV infected. STI, blood history of transfusion, tattoo, body piercing, and previous use of glass syringe were identified in 15.7%, 21.6%, 7.8%, 4.9%, and 49.0% of the sample, respectively. Smoked drugs use was the most frequently reported (6.9%). Additionally, sharp objects use (19.6%) and vertical transmission (15.7%) were the most reported means of HBV infection. Importantly, there was a high frequency of HBV-infected family members among the study sample. In a previous study, conducted in a city geographically and culturally close to Bento Gonçalves (Caxias do Sul), important associations were detected between intrafamilial transmission of HBV,30 a fact that justifies our findings. Evidence also corroborates this type of transmission in Northern Brazil.13 Currently, phylogenetic approaches reinforce the context of intrafamilial transmission in different studies.14,15,31
Over 50% of patients had at least one relative infected with HBV demonstrating the importance of HBV transmission in the family environment, mainly in childhood as previously observed in southern Brazil.30 In addition, there is evidence of a high frequency of HBV infection in siblings (75%) born to HBsAg-positive mothers (p <0.01) as observed in other study.13
Alcohol consumption was also investigated in the present study. We observed that 13.7% of patients had alcohol use disorder by AUDIT scores. Considering the additional damage that alcohol ingestion may produce in patients with chronic hepatitis B, these findings highlight the importance of identifying the pattern of alcohol ingestion in patients with chronic liver diseases.32 In this study, frequencies for illicit drugs use of 6.9% for smoked drugs, 1.9% for sniffed drugs, and 0.9% for injected drugs were observed. Alcohol consumption and drugs use (especially injected) had already been identified as relevant risk factors for HBV infection.10 In Brazil, association between heavy alcohol consumption and HBV infection was previously seen in one region (Southeast), but not in others (Central-West, North, Northeast, and South).10 Further, in our country, illicit drugs use has also not been associated with HBV infection in a nationwide study.33 However, sniffed and inhaled drugs was shown to be associated with HBV in South Brazil in a more recent investigation.10 Finally, in the Southern region of the country (Caxias do Sul City), HBV infection was not associated with neither illicit drugs or alcohol consumption.30
In this study, 15.7% of the sample had a history of STI. This classical risk factor (STI) was not shown to be associated to HBV infection in Southern Brazil in a recent study.30 On the other hand, other studies found an association between the history of STI and infection for HBV in a multicentric population-based study in Central-West, Northeast and Federal Districts of Brazil,33 and in population-based survey in North, Southwest, and South of Brazil.10
The frequency of body piercing and tattoo were 10.6% and 7.8%, respectively. Although these esthetic features have been associated with HBV infection,8,9,33 studies in Southern Brazil have not detected any association.10,30 In this study, 49.0% and 21.6% of HBV-infected people had previous use of glass syringe and blood transfusion, respectively. These factors were shown to be associated with HBV infection in a previous study in Southern Brazil.10,33 Further, in this study, the frequency of share of personal objects in the family environment was of 50.0%. This variable was associated with HBV in other previous studies.13,30,34 Generally, sharing razor, cutlery, face towels, and toothbrush with one infected individual is strongly associated with HBV transmission.10,13,30
Additionally, we evaluated the frequency of HBV genotypes among chronically infected patients in Bento Gonçalves, Southern Brazil. Previous studies reported the major prevalence of genotypes A, D, and F among Brazilians.5,19,21 Our results revealed marked predominance of genotype D, with a minority of patients infected with genotype A. Moreover, no other genotype was detected in the present study. The high prevalence of genotype D is in agreement with studies conducted in Southern Brazil.19,21 However, this high prevalence of genotype D in Southern Brazil differs from the findings of the whole country (A is the most frequent genotype).5 This regional epidemiological difference must be taken into account because of the continental extent of the country.
Phylogenetic studies have been able to trace geographic routes and speed of dissemination of HBV genotypes and subgenotypes.35 Socio-demographic characteristics influence these processes, with the population profiles defining the migration and speed of HBV dissemination.19 These variables are useful for the characterization of risk factors for hepatitis B and definition of the clinical evolution of the disease and responses to antivirals.15,31,36 In the present study, the subgenotype D3 (81.0%) was the most frequent, followed by D2 (14.3%), and only one (4.7%) case of D1. The same scenario of D3 subgenotype was observed in cities with wide Italian immigration in southern19,21 and southeastern Brazil.15 In addition, the predominance of D3 and D2 subgenotypes was reported in the Missiones region of Argentina, which has a high frequency of Eurodescendant inhabitants.36
The results obtained also allow us to observe that D subgenotype samples were divided into seven branches in the phylogenetic tree, of which they have a high genetic identity, probably having the same evolutionary ancestry of South Europe. In this sense, studies have reported that D3 subgenotype is largely prevalent in Italy.37,38 One could than hypothesize that this subgenotype probably emerged from this region to the Southern of Brazil, especially in the historical period of immigration.
HBV genotyping and sequencing studies provide a better understanding of the risk factors for infection, prognosis and treatment for hepatitis B.15,31,35,36 Phylogenetic analyses conducted in different regions of the world have demonstrated that migratory processes influence the distribution of HBV genotypes and subgenotypes.35 In addition, phylogenetic inferences allow the identification of intrafamilial transmission of HBV, due to the similarities of the viral sequences found in relatives sharing the same domestic environments, as observed in different studies.14,15,31 However, future studies that evaluate HBV phylodynamics and phylogeographic will be useful for the understanding of viral migration and transmission routes in Southern Brazil.
In the present study, the most frequently detected amino acid substitutions in the RT enzyme were Y135S (80.9%) and I266V (66.7%). However, there is no evidence in the literature that Y135S and I266V mutations are related to antiviral resistance. Further, LAM-resistant mutation rtM204I was observed in only one patient, but this patient also presented the compensatory mutation rtL180M. The rtA181V, defined as the signature of ADV-resistant mutations, was also observed in another patient. In this sense, is important to identify antiviral-resistant mutations when using nucleos(t)ide analogues therapy.39 Some HBV mutants have the capacity of exhibiting resistance to antivirals, enhanced virulence, facilitated cell attachment or alteration of epitopes which are important in host immune response.40
Particularly, in our study, ethnicity and familial issues seem to have influence in the distribution of genotype D. Approximately 60% of patients reported that the four grandparents were of Italian ancestry, while 18% reported having at least two grandparents of Italian ancestry. These findings support the hypothesis that genotype D may have been disseminated in this region by Italian immigrants who arrived in Rio Grande do Sul in the 19th century,19,21 since there are studies that show high prevalence of this genotype in Southern Europe including Mediterranean countries, especially in Italy.41,42 Different studies suggested that the high prevalence of genotype D in the Southeast of Brazil is associated with Mediterranean immigrants.19,21 Novel studies are necessary to observe if this landscape is indeed a specific epidemiological characteristic of the other counties in the South region of the country.
ConclusionsIn this study, there was a predominance of HBV genotype D (mainly subgenotype D3) among chronically infected patients in the city of Bento Gonçalves, suggesting an association between Italian immigration and the spread of this infection. Moreover, our results suggest an important role of intrafamilial transmission of HBV infection in the study sample.
AuthorshipJ. Paoli, A.C. Wortmann, D. Simon, N.J.R. Fagundes, and V.R. Lunge designed the study and wrote the protocol. J. Paoli, M.G. Klein, A. Cirolini, and B. Godoy managed to recruit participants and performed the lab work. J.M. Wolf, D. Simon, V.R. Lunge, and V.R.Z.B. Pereira performed the data statistical analyses. J.M. Wolf, D. Simon, V.R. Lunge wrote the first draft of the manuscript and contributed to literature review and discussion of results. All authors contributed to and have approved the final manuscript.
FundingThis research was funded by Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq, research grant 559598/2009-2).
Conflicts of interestThe authors declare no conflicts of interest.
The authors would like to thank the patients for their collaboration to make this study possible.