Hanseniasis is a public health concern in developing countries. Although a decrease in the number of new cases in Brazil has been reported, there is a prevalence above that recommended in some regions.
AimsConsidering the goal of the World Health Organization (WHO) to accelerate towards a leprosy-free world from 2020, the aim of this study was to analyze the epidemiological profile and leprosy trends in the city of Cruzeiro do Sul, Acre, Brazil.
MethodsThis retrospective cohort study analyzed the epidemiology and trends of hanseniasis between 2005 and 2018, monitoring socioeconomic and clinical epidemiological variables obtained from the Information System of Notifiable Diseases of Hanseniasis (SINAN) database.
ResultsA total of 422 cases of hanseniasis (284 male, 138 female) were included. The questionnaire of six patients was incomplete. The highest number of cases (89) was recorded in 2006 (11.7/10,000 inhabitants). The borderline clinical form was most common, with 45.4% of cases. Throughout the historical series, the rate of annual percentage change in the detection of new cases and cases with grade 2 disability showed a decreasing profile, at −13.9 [95% CI: −19.1, −8.2] and −13.1 [95% CI: −21.8, −5.5], respectively. The same rates were observed in patients below 15 years of age.
LimitationsThis study reflects the scenario in one reference center and data were obtained retrospectively.
ConclusionsThe incidence of hanseniasis in this reference center is declining gradually; however, the indicators show active disease transmission and late diagnosis.
Hanseniasis is a major public health problem in developing countries.1 In 2015, the Pan American Health Organization and the World Health Organization (PAHO/WHO) presented the "Global Strategy for Leprosy 2016–2020,” proposing a leprosy-free world.2,3 New cases of hanseniasis have decreased in the past 10 years, according to WHO, reaching an affected population of 208,641 new cases in 2018 against 244,796 in 2009. A new case detection rate of 2.74 per 100,000 population in 2018 was reported. However, some countries remain endemic and specific regions, such as Chhattisgarh (India), Pará (Brazil), and Madura (Indonesia),4 still account for 80% of cases worldwide.4,5
In 2018, Brazil had a new case detection rate of 13.7 per 100,000 inhabitants, higher than in India (8.9/100,000 inhabitants) and Indonesia (6.36/100,000 population).4 In north Brazil, the rate was 31.95/100,000 inhabitants.5 Although hanseniasis is present in 26 out of 34 countries of the Americas, 25 of them eliminated the disease as a public health problem, except for Brazil, responsible for 93% of new cases in 2018.2 The city of Cruzeiro do Sul, Acre, is a reference center for hanseniasis control in the northern region of the country. Thus, the aim of this study was to analyze the epidemiological profile and hanseniasis trends from 2005 to 2018, in addition to the clinical characteristics of the patients.
Materials and methodsThe present temporal ecology study consolidated data from 422 hanseniasis cases treated at the Sanitary Dermatology Hospital in a municipality with 87,673 inhabitants. Only resident individuals with a confirmed diagnosis of hanseniasis between January 1, 2005, and December 31, 2018, and recorded in the Information System of Notifiable Diseases (SINAN) database6 were included in the study. The following socioeconomic variables were obtained: sex, age group, educational level, race/color, and occupation. Clinical variables included: mode of detection of new cases: spontaneous demand, contact examination and collectivity exam. Spontaneous demand corresponded to patients going voluntarily to the health unit; collective examination was referred to an active search for the diagnosis. Hanseniasis was classified according to clinical form as undetermined, tuberculoid, borderline and lepromatous; degree of physical disability (0 or absence of disability, I, II and III), and operational classification (paucibacillary, multibacillary), to establish the therapy (Madrid/Mitsuda (1953) and Ridley & Jopling (1966).9 For the epidemiological characterization of hanseniasis, the following indicators were calculated: new case detection rate per 100,000 inhabitants (measures the strength of morbidity, magnitude and endemic profile); new case detection rate per 100,000 inhabitants, in children under 15 years of age (measures the strength of recent transmission and its profile); rate of new hanseniasis cases with grade 2 physical disability (severe sequelae causing deformities) per 1,000,000 inhabitants and the proportion of cases with grade 2 physical disability among the new cases (assesses the effectiveness of early detection activities).8
Chi-square test was applied to assess the association between two variables. For temporal trends, regression analysis was performed with joinpoint regression, version 4.6.0., which tests whether an apparent change in trend is statistically significant. To characterize the trends, we calculated the annual percentage rate change (APC), given by 100[exp(β1) −1], where β1 is the slope from the regression model on a log scale. The p-value (two-sided test) checked whether the true APC is zero, and was calculated based on a t distribution.
In addition to APC, the average annual percent change (AAPC) was also calculated by the weighted average of the APCs from the joinpoint model, with the weights equal to the length of the APC interval. If an AAPC lies entirely within a single joinpoint segment, the AAPC is equal to that segment's APC. The trend was classified as increasing for positive values (annual percentage change - APC), decreasing for negative values, and stationary when there was no significant difference between the observed value and zero. The level of significance adopted in all analyses was 5% (p<0.05).
This study was approved by the Research Ethics Committee 5009-Clinical Hospital of Acre-HCA/FUNDHACRE (CAAE: 58066816.5.0000.5009).
ResultsSocioeconomic profileThe study included 422 cases of hanseniasis (284 male, 138 female), with a mean of 30 cases/year. The questionnaire of six patients was incomplete. The highest percentages was reported for race, afro-Brazilians (85.1%), and the age group of 30–59-years (45.1%). A high percentage of the cases reported neither education level (37%) nor occupation (38.2%). Of those who did report, the highest percentage was incomplete primary (36.5%) education, followed by illiteracy (11.6%). In relation to occupation, men were engaged in agriculture (31.3%) and women were housewives (22.5%), with a statistical association between sex and occupation (p<0.05).
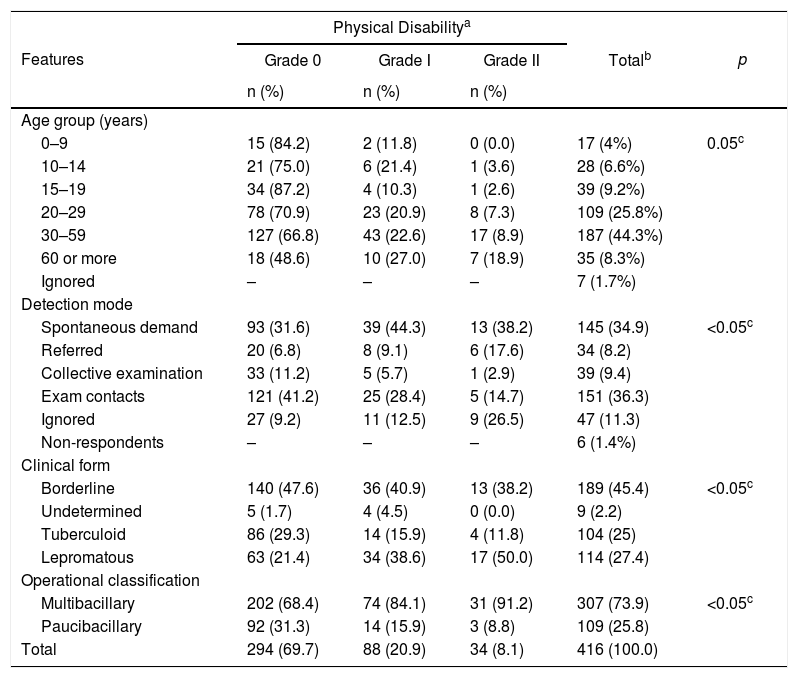
Clinical-epidemiological profileContact examination and spontaneous demand were the most common forms of detection among notified patients (Table 1). Regarding clinical form, the borderline was the most common, followed by lepromatous and tuberculoid forms (Table 1). Higher percentages of no physical disability (grade 0) were seen with borderline (47.6%) and tuberculoid (29.3%) clinical forms. For the lepromatous clinical form, considered the most severe presentation, 50% of the cases were classified as grade two, showing a significant association between both variables (Table 1). A significant association between sex and clinical form or degree of physical disability was observed, with more severe disease cases (lepromatous form and grade 2 disability) among males (results not shown). The multibacillary form represented 74% of hanseniasis cases. Of these, 91.2% presented grade 2 disability (Table 1). The highest percentage of cases with no disability occurred for patients aged ≤19 years and in grade 2 disability for patients aged 60 years or older.
Age groups distribution, detection mode, clinical form, and operating class according to physical disability among leprosy records in Cruzeiro do Sul, Acre, 2005–2018.
| Features | Physical Disabilitya | p | |||
|---|---|---|---|---|---|
| Grade 0 | Grade I | Grade II | Totalb | ||
| n (%) | n (%) | n (%) | |||
| Age group (years) | |||||
| 0–9 | 15 (84.2) | 2 (11.8) | 0 (0.0) | 17 (4%) | 0.05c |
| 10–14 | 21 (75.0) | 6 (21.4) | 1 (3.6) | 28 (6.6%) | |
| 15–19 | 34 (87.2) | 4 (10.3) | 1 (2.6) | 39 (9.2%) | |
| 20–29 | 78 (70.9) | 23 (20.9) | 8 (7.3) | 109 (25.8%) | |
| 30–59 | 127 (66.8) | 43 (22.6) | 17 (8.9) | 187 (44.3%) | |
| 60 or more | 18 (48.6) | 10 (27.0) | 7 (18.9) | 35 (8.3%) | |
| Ignored | – | – | – | 7 (1.7%) | |
| Detection mode | |||||
| Spontaneous demand | 93 (31.6) | 39 (44.3) | 13 (38.2) | 145 (34.9) | <0.05c |
| Referred | 20 (6.8) | 8 (9.1) | 6 (17.6) | 34 (8.2) | |
| Collective examination | 33 (11.2) | 5 (5.7) | 1 (2.9) | 39 (9.4) | |
| Exam contacts | 121 (41.2) | 25 (28.4) | 5 (14.7) | 151 (36.3) | |
| Ignored | 27 (9.2) | 11 (12.5) | 9 (26.5) | 47 (11.3) | |
| Non-respondents | – | – | – | 6 (1.4%) | |
| Clinical form | |||||
| Borderline | 140 (47.6) | 36 (40.9) | 13 (38.2) | 189 (45.4) | <0.05c |
| Undetermined | 5 (1.7) | 4 (4.5) | 0 (0.0) | 9 (2.2) | |
| Tuberculoid | 86 (29.3) | 14 (15.9) | 4 (11.8) | 104 (25) | |
| Lepromatous | 63 (21.4) | 34 (38.6) | 17 (50.0) | 114 (27.4) | |
| Operational classification | |||||
| Multibacillary | 202 (68.4) | 74 (84.1) | 31 (91.2) | 307 (73.9) | <0.05c |
| Paucibacillary | 92 (31.3) | 14 (15.9) | 3 (8.8) | 109 (25.8) | |
| Total | 294 (69.7) | 88 (20.9) | 34 (8.1) | 416 (100.0) | |
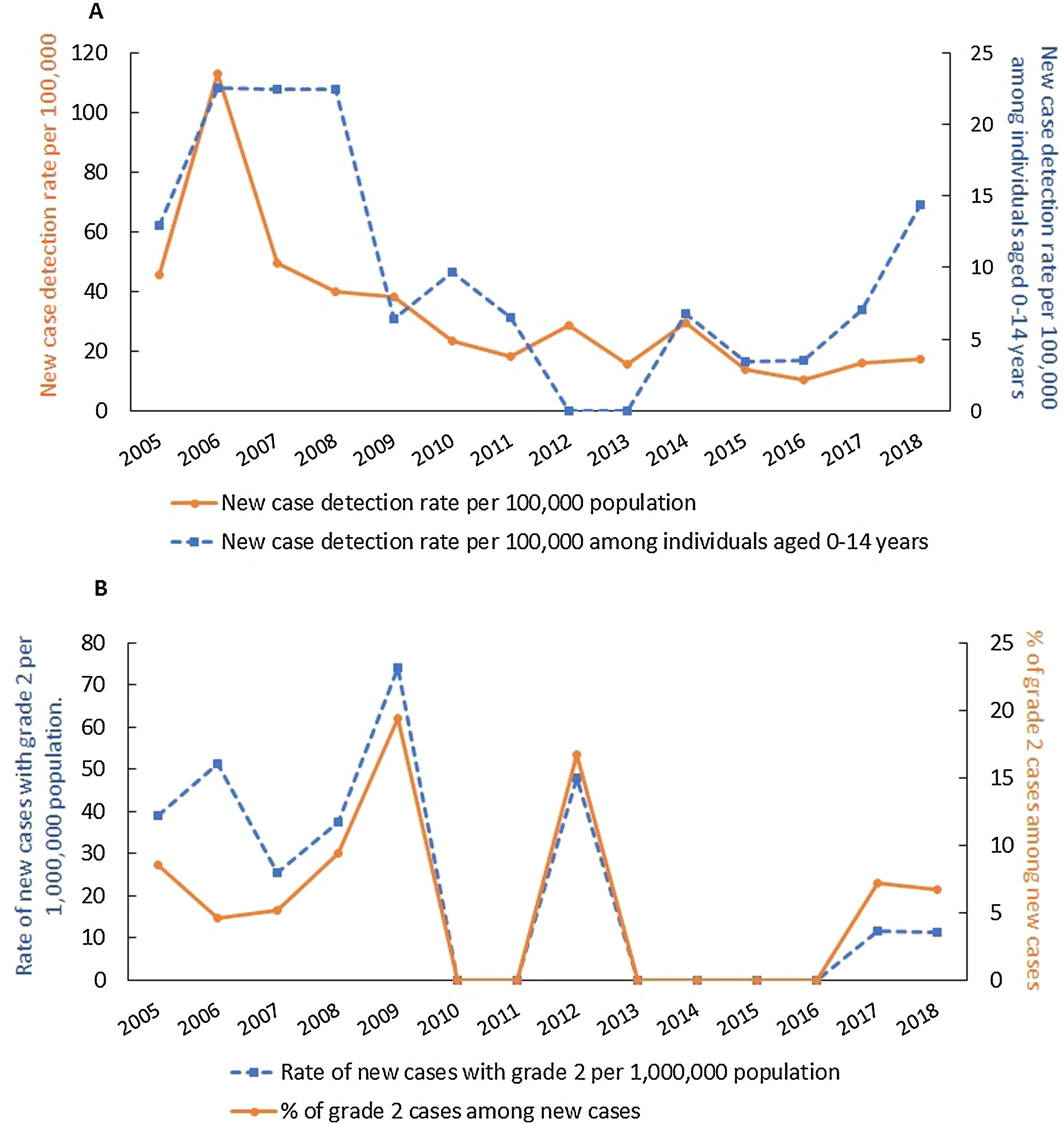
A total of 422 new cases were reported during the study period, decreasing from 35 in 2005 to 15 in 2018, corresponding to new cases detection rates of 45.5/100,000 and 17.1/100,000 inhabitants, respectively (Fig. 1A). The highest number of cases registered (89) and new cases (88) were recorded in 2006, resulting in a prevalence and new case detection rates of 11.7/10,000 inhabitants and 115.6/100,000 inhabitants, respectively (Fig. 1A). The average prevalence rate was 3.7 per 10,000 inhabitants and the new case detection rate was 32.8 per 100,000 inhabitants (Fig. 1A).
The new cases detection rate showed a decreasing trend throughout the historical series, with APC (Annual Percent Change) of −13.9 (95% CI: −19.1% to −8.2%). Of the total new cases, 45 (10.8%) were children <15 years, resulting in an average new cases detection rate of 9.9/100,000. Throughout the historical series (2005–2018), AAPC (Average Annual Percent Change) was −4.2 (95% CI: −13.1% to 3.6%), indicating a stationary trend for new cases detection rate among children under 15 years of age (Fig. 1A). The total number of new cases with grade 2 disability (G2D) was 24, with the highest number of cases (6) seen in 2009 (Fig. 1B). At the end of the reporting year, one G2D case was observed among new cases, resulting in a rate of 11.4 per million and a percentage of 6.7% (Fig. 1B). The rate of new cases with G2D showed a decreasing trend (APC: −13.1%, 95% CI: −21.8% to −5.5%) and the proportion of cases with grade 2 disability among new cases remained stable throughout the study period (APC: 0.5%, 95% CI: −9.2% to 11.2%) [Fig. 1B].
DiscussionHistorically, various attempts to eradicate, eliminate and control hanseniasis have been proposed over the centuries, such as compulsory isolation and use of dapsone, until the establishment of polychemotherapy (1982). In 1986, WHO implemented the strategy to eliminate hanseniasis up aiming an 80% reduction by the year 2000. Neither Brazil nor India and Indonesia achieved this goal.3,9 In 2016, WHO launched yet another proposal, with zero new child cases with G2D by 2020.3,4 The municipality of Cruzeiro do Sul is located in an endemic region for hanseniasis in Brazil, and then we foresaw an excellent opportunity to evaluate several aspects that could explain the high prevalence of the disease there. Although we observed a general trend towards declining hanseniasis indicator rates, the prevalence rate (1.93/10,000 inhabitants in 2018) remains higher than in countries not considered endemic for hanseniasis.13
Epidemiological indicators can monitor the progress of leprosy elimination as a public health problem. The decreasing trend in the rate of detection of new cases accompanied by a significant reduction in the rate of new cases with grade 2 physical disability, indicates a reduction in the strength of morbidity and magnitude of the endemic disease.8 However, new cases detection rate stability among children under 15 years of age indicates active transmission and early exposure to the bacillus and hampers programs to control the disease.4,8,10–12 Also, the stable trend in the proportion of new cases with G2D suggests that late diagnosis remain.10,11,13 Based on these indicators, one can conclude that leprosy transmission remains a significant public health problem in the municipality of Cruzeiro do Sul.
Despite the reduction in the detection rate of new cases, the situation moved from hyperendemicity (≥40.0/100 thousand inhabitants) in 2005 to high endemicity (10.00–19.99/100 thousand inhabitants) in 2018, according to WHO standards, showing the severity of the problem. Also, the rate of detection of new cases (17.1/100,000 inhabitants) in 2018 was higher compared to the Brazilian rate (13.7/100,000 inhabitants).
The proportion of new cases with grade 2 physical disability has been used to assess early disease diagnosis, since cases diagnosed with some degree of physical disability indicate late diagnosis even before the start of polychemotherapy. This indicator did not follow the downward trend in detecting new cases, once again indicating late diagnosis and a probably hidden prevalence of the disease.
Late diagnosis reflects the inability of health services in the municipality to catch and treat all cases early, leaving people without treatment.8,10 Financial support was received by the municipality at the end of 2005 for specific actions against hanseniasis.9,14 Field teams could perform active searching, do physical exams in the community, and diagnose initial clinical forms, such as paucibacillary (tuberculoid). Treatment time was shortened, decreasing risk of transmission and disability. However, the investment finished from 2006 onwards and interrupted the previous approach. Spontaneous demand has been the primary detection method and typically identifies patients with multibacillary clinical forms (borderline and lepromatous) and grade 2 disability. These observations confirm the indicators profile, reducing case numbers and inefficient early case detection. This situation leads to underreported cases.
As hanseniasis is characterized as a contact disease that is insidious and painless, leading to chronicity and showing a long latency period, late diagnosis is common.15 Demand for specific attention by patients with suspected hanseniasis is influenced by persistent stigma.14 More consistent work is required to address this situation, involving screening and physical examination of all contacts over long periods, to show the real trends in detection and improve early diagnosis to eliminate the disease.8,16,18
Patients’ socioeconomic profile consisted mostly of young adult men in productive and reproductive age, illiterate or with incomplete primary education, living in urban areas but working in agriculture. The constant migration from rural to urban areas results in an unequal distribution of cases in the Brazilian territory, with urban concentration.19 Hanseniasis is considered endemic in low-income populations and the North, Northeast and Midwest regions of Brazil where the highest rates of illiteracy and social inequality is seen, around 18.5%, 11.1% and 7.0%, respectively.9 Cruzeiro do Sul, being a municipality inserted in this context, corroborates the description above. The endemicity of the disease also reflects the imbalance of health care in different regions of our country.6,20,21 In Caxias (Maranhão), northeast of Brazil, Lima et al. (2009) observed a similar clinical and epidemiological profile identified here; however, there was an increasing trend towards disease endemicity.19 In contrast, in the northeastern region (Fortaleza), the results suggested a late disease diagnosis.7,10,17,
This study has limitation as it reports results of a single reference center; however, it represents an area of high endemicity in our country. Data reported here were from the official government registry, but obtained retrospectively.
The WHO has recognized that certain developing countries have to integrate better the control programs of neglected tropical diseases (NTD), improve management of these diseases, reduce disparities, and increase the efficiency of services to reach a higher percentage of the population.22 We are moving forward; however, there is still a long way to go. The long-term control of hanseniasis has to re-consider better forms of detection and examination of contacts.
Conflicts of interestThere are no conflicts of interest regarding this manuscript.
Financial disclosureThis study was supported by a multi-institutional agreement (n. 007/2015/SESACRE-UFAC-FMABC) for post-graduation course.
We thank the staff of Sanitary Dermatology Hospital, Cruzeiro do Sul, for its collaboration during the development of the research.