Multidrug-resistant tuberculosis (MDR-TB) represents a significant impact in transmission, outcome, and health costs. The World Health Organization recommends implementation of rapid diagnostic methods for multidrug-resistance detection. This study was performed to evaluate the frequency of pre- and extensively drug resistant tuberculosis (pre-XDR-TB and XDR-TB) among MDR-TB patients, the pattern of resistance mutations for fluoroquinolones and the clinical outcome. Adult patients followed at a Brazilian regional reference center for TB, from January 2013 to June 2019 were included. Stored Mycobacterium tuberculosis (Mtb) cultures were recovered, the DNA was extracted, and the susceptibility test was performed using the line probe assay for second line antimycobacterial drugs, Genotype MTBDRsl version 2.0 (Hain Lifescience, CmbH, Germany). Among 33 MDR-TB included patients, we diagnosed XDR-TB or pre-XDR in five (15%) cases. Of these, mutations related to fluoroquinolones resistance were observed in four Mtb isolates, including one who had no phenotypic resistance profile. In two other patients with phenotypic resistance to ofloxacin, genotypic resistance was not found. Case fatality rate was 60% in pre/XDR-TB group, compared to 3.6% in the remaining of patients. This study observed few cases of pre-XDR and XDR-TB among a MDR-TB cohort. Phenotypic and genotypic assays presented good agreement. Clinical outcome was more favorable for patients with susceptibility to fluoroquinolones and injectable drugs.
The World Health Organization (WHO) recommends three pillars for tuberculosis (TB) control and eradication: integrated patient-centered care and prevention; bold policies and support systems, especially for vulnerable populations; and intensification in research and innovation1 with emphasis on resistance detection and new diagnostic assays.2 Multidrug-resistant TB (MDR-TB), (resistance to isoniazid [INH] and rifampicin [RIF]), impacts transmission, outcome, and health costs. MDR-TB occurs, in about, 20.5% of retreated patients and in 3.5% of new TB cases.2 Besides RIF and INH resistance, cases with extensively drug-resistant TB (XDR-TB) have also resistance to fluoroquinolones (FQ) and second-line injectable drugs (pre-XDR-TB). About 10.2% of MDR-TB cases in São Paulo state presented XDR-TB or pre-XDR-TB.5
Universal access to drug susceptibility testing (DST) represents the standard of care for TB. Phenotypic DSTs (pDST) are based in mycobacterial cultures and require significant infrastructure with slow turnaround time. Considering this scenario, the focus should be on rapid and accurate MDR-TB detection with molecular assays. Paradigms have been broken in TB diagnosis, mainly in the last decade.3,4 Currently, the molecular rapid assay Xpert MTB/RIF (Xpert, Cepheid, Sunnyvale, California) is available in developing countries, including in Brazilian Public Health Care System (SUS) since 2016.6–8 This real-time polymerase chain reaction (PCR) based assay detects the Mycobacterium tuberculosis (Mtb) and resistance to RIF, the backbone of TB regimens. Several genotypic DST have been evaluated to improve the adequacy of M/XDR-TB regimens.
Molecular hybridization assays based on strips, line probe assays (LPAs) are useful and accurate in different settings and infectious diseases. The rationale of these assays is that specific probes for naive and mutant alleles of resistance genes are conjugated with PCR products.9 Commercial LPA is available for detection of MDR-TB (Genotype MTBDRplus assay [Hain Lifescience, CmbH, Germany]). This product has shown 92.2% sensitivity and 99.3% specificity,10 but not for INH. More recently, a hierarchical cascade for Mtb resistance diagnosis has been proposed using LPA, aiming to provide quick decisions on TB treatment.11
According to the WHO, LPAs assays for Mtb resistance detection are recommended to reduce the time of MDR-TB diagnosis. LPAs are applicable for patients with signs and symptoms of TB with AFB positive in sputum directly from clinical specimens.12 For second-line drugs, LPA has shown sensitivity around 97% and 100% specificity even in smear sputum samples.9 This version detects resistance mutations related to FQ, second-line injectable drugs aminoglycosides (amikacin, kanamycin), and glycopeptides (capreomycin).9 FQ resistance is investigated in regions of 83 and 96 codons of gyrA and 538 and 540 of gyrB, representing more than 70% of resistance mutations.13,14 Regarding aminoglycosides, target regions are the codons of rrs gene (1401, 1402, 1484) and some positions of eis gene.15
Herein we evaluate the frequency of pre-XDR-TB and XDR-TB among MDR-TB patients, in addition to assess the pattern of resistance mutations for FQ and the clinical outcome in a regional reference center for MDR-TB.
Material and methodsDesignA cross-sectional study involving MDR-TB patients, from January 2013 to June 2019. Setting: a tertiary care hospital with a regional MDR-TB outpatient clinic, reference for 6,000,000 inhabitants with a TB incidence rate of 30 cases per 100,000 inhabitants.
PopulationPatients with MDR-TB (resistance to RIF and INH) followed in our regional MDR-TB outpatient clinic were eligible for the study. Inclusion criteria: adults aged 18 years old or higher; diagnosis of pulmonary or extra-pulmonary tuberculosis confirmed by positive culture for Mtb; detection of RIF and INH resistance by BD BACTEC-MGIT automated system (BD LifeSciences, New Jersey) performed by the central reference public health laboratory of São Paulo state (Adolfo Lutz Institute). Exclusion criteria: patients whose isolates from Mtb were not available. Ethical Research Committee of the State University of Campinas approved the study.
MethodsEpidemiological and clinical data from clinical records and database of reported TB cases were collected. Isolates were selected from a microbiological collection of the Clinical Pathology Department. Mtb isolates frozen were recovered in Löwenstein–Jensen medium incubated at 36°C.16
If the culture was negative after 60 days of incubation, it was considered inadequate for study. Laboratory procedures consisted of the following steps: first, Mtb DNA was extracted by thermal lysis17; subsequently DST was conducted using line probe assay for second-line antimycobacterial drugs (LPAsl), Genotype MTBDRsl version 2.0 (Hain Lifescience, CmbH, Germany). In brief, this technique includes DNA amplification by PCR, hybridization with specific probes, detection, and interpretation of results compared with standardized strips.
AnalysisTwo groups were defined according to DST results: the first was MDR-TB cases and the second pre-XDR-TB & XDR-TB cases. Groups were defined on basis of resistance to FQ and/or injectable second-line drugs using a line probe assay for second-line drugs. The outcome measure was clinical follow-up classified as favorable (cure or clinical improvement) or unfavorable (death or treatment default). Results were analyzed using Epi Info version 7. Univariate analysis was performed for comparison between groups. For categorical variables, Fisher's exact test was used when appropriate. Non-categorical variables were compared by Wilcoxon–Mann–Whitney. A p-value less than 0.05 was considered statistically significant.
ResultsDuring the study period 33 patients with MDR-TB were eligible for this study. Of these, five (15.1%) patients were detected with pre-XDR or XDR-TB. Two patients already had XDR phenotypic resistance profiles and three others, in addition to rifampicin and isoniazid, had additional resistance to ofloxacin, pre-XDR-TB.
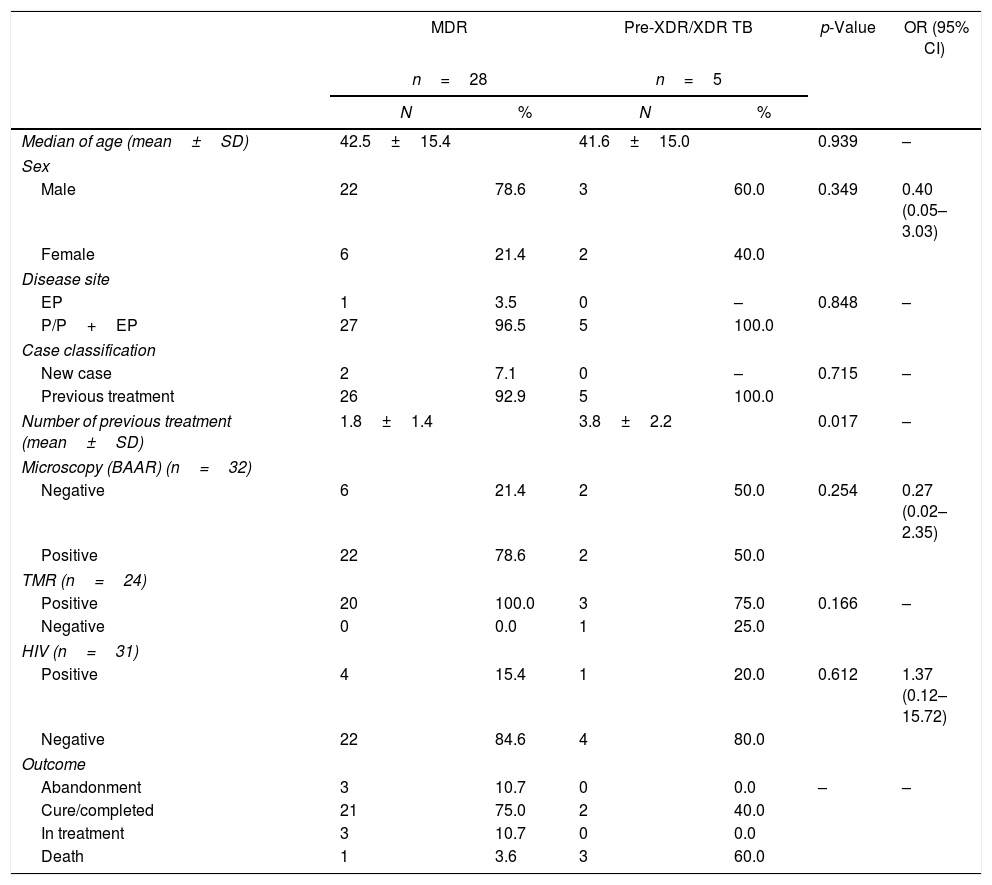
Patients with pre-XDR-TB or XDR-TB had pulmonary forms of the disease. All patients had a history of previous TB treatment; moreover, two of them had seven courses of previous treatments. On the other hand, the MDR group had an average of 1.8 previous TB treatments. The number of previous treatments was statistically different between the two groups. Despite the onset of the disease and identification of extensive resistance, two patients had negative smear microscopy. Only one patient with pre-XDR-TB or XDR-TB resistance profile was HIV-infected (Table 1).
Epidemiological and clinical characteristics according to the resistance pattern.
| MDR | Pre-XDR/XDR TB | p-Value | OR (95% CI) | |||
|---|---|---|---|---|---|---|
| n=28 | n=5 | |||||
| N | % | N | % | |||
| Median of age (mean±SD) | 42.5±15.4 | 41.6±15.0 | 0.939 | – | ||
| Sex | ||||||
| Male | 22 | 78.6 | 3 | 60.0 | 0.349 | 0.40 (0.05–3.03) |
| Female | 6 | 21.4 | 2 | 40.0 | ||
| Disease site | ||||||
| EP | 1 | 3.5 | 0 | – | 0.848 | – |
| P/P+EP | 27 | 96.5 | 5 | 100.0 | ||
| Case classification | ||||||
| New case | 2 | 7.1 | 0 | – | 0.715 | – |
| Previous treatment | 26 | 92.9 | 5 | 100.0 | ||
| Number of previous treatment (mean±SD) | 1.8±1.4 | 3.8±2.2 | 0.017 | – | ||
| Microscopy (BAAR) (n=32) | ||||||
| Negative | 6 | 21.4 | 2 | 50.0 | 0.254 | 0.27 (0.02–2.35) |
| Positive | 22 | 78.6 | 2 | 50.0 | ||
| TMR (n=24) | ||||||
| Positive | 20 | 100.0 | 3 | 75.0 | 0.166 | – |
| Negative | 0 | 0.0 | 1 | 25.0 | ||
| HIV (n=31) | ||||||
| Positive | 4 | 15.4 | 1 | 20.0 | 0.612 | 1.37 (0.12–15.72) |
| Negative | 22 | 84.6 | 4 | 80.0 | ||
| Outcome | ||||||
| Abandonment | 3 | 10.7 | 0 | 0.0 | – | – |
| Cure/completed | 21 | 75.0 | 2 | 40.0 | ||
| In treatment | 3 | 10.7 | 0 | 0.0 | ||
| Death | 1 | 3.6 | 3 | 60.0 | ||
MDR, multidrug-resistant; Pre-XDR/XDR TB, pre- and extensively-resistant.
Overall death rate was in 12%; higher lethality was registered in patients with pre-XDR and XDR-TB (60.0%) compared to MDR-TB (3.6%). Furthermore, 75.0% of MDR patients have completed treatment against 40.0% of the other group.
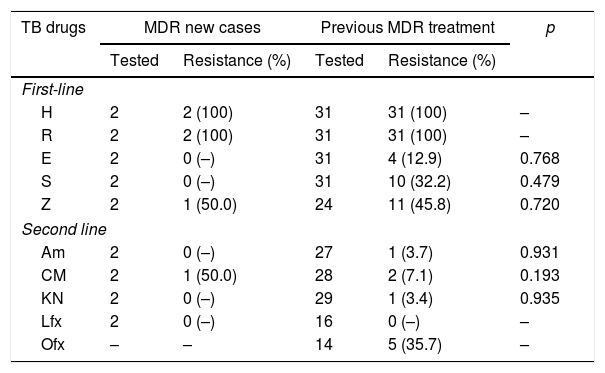
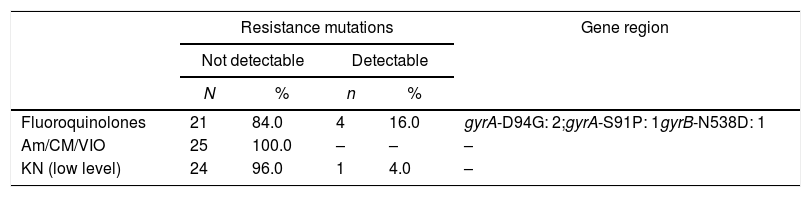
All 33 isolates from MDR-TB patients were tested for first-line drugs with pDST, except for pyrazinamide. For the second-line drugs, the evaluation was available for 32 patients; ofloxacin resistance was more prevalent, followed by capreomycin (Table 2). Twenty-five isolates of Mtb underwent LPA: four isolates showed FQ resistance genes mutations pattern and one M. tuberculosis isolate had low level resistance to kanamycin (eis gene). For this investigation, three mutations associated with the gyrA gene were found and only one for gyrB gene: gyrA D94G in two patients, gyrA S91P and gyrB N538D (Table 3).
Phenotypic drug susceptibility tests for first and second-line TB drugs.
| TB drugs | MDR new cases | Previous MDR treatment | p | ||
|---|---|---|---|---|---|
| Tested | Resistance (%) | Tested | Resistance (%) | ||
| First-line | |||||
| H | 2 | 2 (100) | 31 | 31 (100) | – |
| R | 2 | 2 (100) | 31 | 31 (100) | – |
| E | 2 | 0 (–) | 31 | 4 (12.9) | 0.768 |
| S | 2 | 0 (–) | 31 | 10 (32.2) | 0.479 |
| Z | 2 | 1 (50.0) | 24 | 11 (45.8) | 0.720 |
| Second line | |||||
| Am | 2 | 0 (–) | 27 | 1 (3.7) | 0.931 |
| CM | 2 | 1 (50.0) | 28 | 2 (7.1) | 0.193 |
| KN | 2 | 0 (–) | 29 | 1 (3.4) | 0.935 |
| Lfx | 2 | 0 (–) | 16 | 0 (–) | – |
| Ofx | – | – | 14 | 5 (35.7) | – |
H, isoniazid; R, rifampin; E, ethambutol; S, streptomycin; Z, pyrazinamide; Am, amikacin; CM, capreomycin; Kn, kanamycin; Lfx, levofloxacin; Ofx, ofloxacin.
Results of line probe assay for second-line drugs among patients with MDR-TB.
| Resistance mutations | Gene region | ||||
|---|---|---|---|---|---|
| Not detectable | Detectable | ||||
| N | % | n | % | ||
| Fluoroquinolones | 21 | 84.0 | 4 | 16.0 | gyrA-D94G: 2;gyrA-S91P: 1gyrB-N538D: 1 |
| Am/CM/VIO | 25 | 100.0 | – | – | – |
| KN (low level) | 24 | 96.0 | 1 | 4.0 | – |
KN, kanamycin; Am, amikacin; CM, capreomycin; VIO, viomicin.
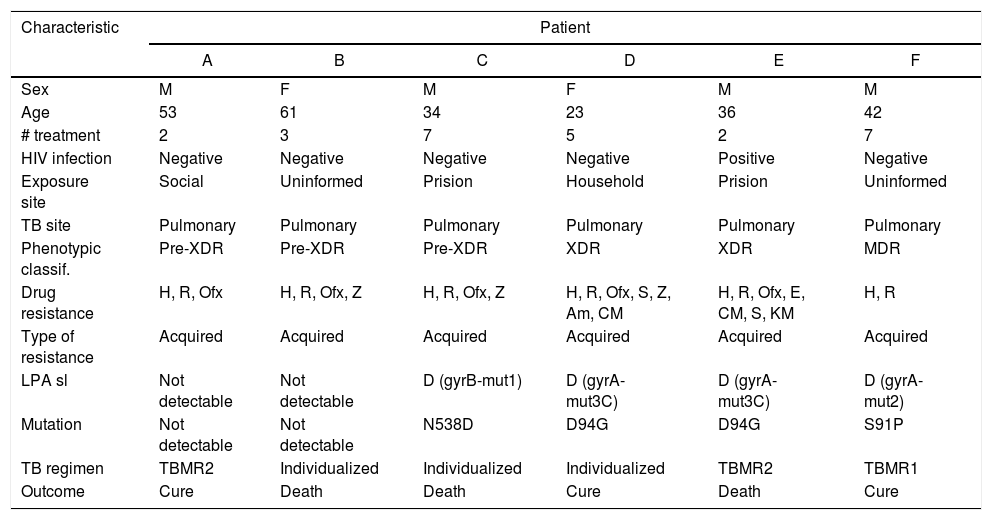
Three cases of disagreement between LPAsl and pDST were as follows: one with FQ resistance gene mutation pattern, but susceptible in the phenotypic assay, and other two patients with a phenotypic pre-XDR result without any genotypic resistance pattern on LPA (Table 4).
Characteristics of patients with pre-XDR-TB and XDR-TB.
| Characteristic | Patient | |||||
|---|---|---|---|---|---|---|
| A | B | C | D | E | F | |
| Sex | M | F | M | F | M | M |
| Age | 53 | 61 | 34 | 23 | 36 | 42 |
| # treatment | 2 | 3 | 7 | 5 | 2 | 7 |
| HIV infection | Negative | Negative | Negative | Negative | Positive | Negative |
| Exposure site | Social | Uninformed | Prision | Household | Prision | Uninformed |
| TB site | Pulmonary | Pulmonary | Pulmonary | Pulmonary | Pulmonary | Pulmonary |
| Phenotypic classif. | Pre-XDR | Pre-XDR | Pre-XDR | XDR | XDR | MDR |
| Drug resistance | H, R, Ofx | H, R, Ofx, Z | H, R, Ofx, Z | H, R, Ofx, S, Z, Am, CM | H, R, Ofx, E, CM, S, KM | H, R |
| Type of resistance | Acquired | Acquired | Acquired | Acquired | Acquired | Acquired |
| LPA sl | Not detectable | Not detectable | D (gyrB-mut1) | D (gyrA-mut3C) | D (gyrA-mut3C) | D (gyrA-mut2) |
| Mutation | Not detectable | Not detectable | N538D | D94G | D94G | S91P |
| TB regimen | TBMR2 | Individualized | Individualized | Individualized | TBMR2 | TBMR1 |
| Outcome | Cure | Death | Death | Cure | Death | Cure |
WHO has reported that 17% of patients with MDR resistance profile also had additional resistance to FQ.18 In our study, despite the limited number of patients evaluated, a similar prevalence was observed. Gallo et al. found higher rates (26%) of pre-XDR-TB among MDR-TB patients in São Paulo state treated from 2006 to 2013.8
Pre-XDR-TB or XDR-TB cases had many previous treatments as demonstrated by others.19 In this group, with multiple exposures to antimycobacterial regimens, the LPAsl represented a rapid alternative to define the appropriate therapy, together with pDST as proposed by Pan American Health Organization.11 The rapid recognition of FQ resistance demands the incorporation of new drugs like linezolid, bedaquiline, and delamanid into the regimen.
Good agreement of 88% was demonstrated between LPAsl and pDST in our study. Others have reported favorable diagnostic performance of LPAsl. In a multicentric cohort, involving 353 MDR-TB patients, the sensitivity and specificity of LPAsl for FQ compared to phenotypic DST was 80.5% and 100%, respectively.20 In another report, LPA (MDR and sl) and pDST results agreed in 228 of 243 cases (94%).21
Discordant results were observed in three cases: in two false negatives and in one false positive, considering the pDST as the reference standard. Ideally, potential causes of the discrepancies should be analyzed by whole genome sequencing and minimum inhibitory concentration (MIC) determinations. Of note, some authors have considered that pDST likely underestimates the true rate of resistance for key drugs due to high critical concentrations. Multicentric studies should evaluate adequate clinical and epidemiological MIC breakpoints for FQ considering clinical outcomes. In our study, the rate of favorable clinical follow-up was higher among patients with FQ susceptibility compared to FQ resistance. Regarding cross-resistance between different FQ, some in vitro data have shown that Mtb strains have different susceptibility patterns when comparing old and new generation, as well as different MICs.22
In addition, our findings showed genotyping patterns associated with high resistance. Some authors have demonstrated that the MIC for all FQ in isolates that carried gyrA-D94G or gyrB-N538D had significantly higher resistance levels than those isolates with different genotyping.23 A systematic review has demonstrated that gyrA gene mutations are more prevalent in different countries.13
Our study has some limitations: a small number of pre-XDR and XDR-TB was included. Therefore, MICs were not measured, and samples had not undergone genetic sequencing to explore new mutations, which could explain the absence of resistance genotype in LPAsl in correspondence to phenotypic evaluation.
In conclusion, this study observed a few cases of pre-XDR and XDR-TB among an MDR-TB cohort. In some strains, the disagreement was observed between phenotypic methods and LPAsl. Clinical outcome was more favorable for patients with susceptibility to FQ. Evaluation of larger Brazilian cohorts is warranted.
Conflicts of interestThe authors declare no conflicts of interest.