We report a patient with fungal keratitis caused by a multiresistant Fusarium solani in a tertiary care hospital located in southern Brazil. A 55-year-old man with a history of ocular trauma presented with keratitis in left eye. The patient has a complicated clinical course and failed to respond to local and systemic antifungal treatment, and required eye enucleation. Despite multiple topical, intraocular and systemic antifungal treatments, hyphal infiltration persisted in the corneal transplant causing continuous recurrences. The cultures of corneal biopsy scrapings were positive for Fusarium spp. The organism was identified to species level by multi-locus sequencing for translation elongation factor 1 alpha (EF-1α), and RNA polymerase II subunit (RPB2). In vitro antifungal susceptibility testing of the isolate by the broth microdilution method, according to CLSI M38-A2, disclosed susceptibility to natamycin and resistance to amphotericin B, voriconazole, itraconazole and fluconazole. Considering previous unsuccessful antifungal treatments due to multiple drug resistance, the eye was enucleated. Our case report illustrates that management of fungal keratitis remains a therapeutic challenge. Optimal treatment for F. solani infection has not yet been established and should include susceptibility testing for different antifungal agents.
Fusarium keratitis appears as ulcerative lesions and is usually managed using topical antifungal medications, occasionally integrated with subconjunctival injections, although therapeutic keratoplasty may be necessary for patients whose corneal infection persists.1 The species most commonly associated with human infections is F. solani. Fusarium keratitis might progress to deep extensive infection with perforation and malignant glaucoma, which might destroy the eye in a few weeks. Management of Fusarium keratitis is often difficult considering that Fusarium species exhibit broad resistance to the spectrum of antifungals currently available, including amphotericin B, azoles, echinocandins, and terbinafine, which typically show high MICs in vitro testing.2
We describe the clinical course and management of a patient with severe Fusarium keratitis. The patient failed to respond to antifungal treatment and required enucleation surgical procedure.
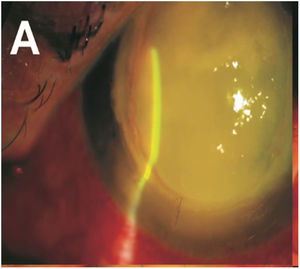
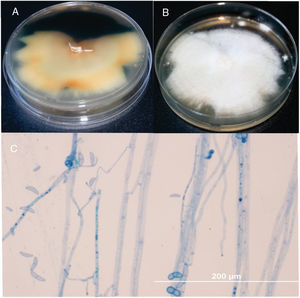
A 55-year-old man with a history of ocular trauma on the left eye 10 days ago presented with keratitis in left eye for ophthalmologic evaluation. The patient had a corneal transplant for keratoconus on the left eye 32 years ago. After the ocular trauma, the patient developed a corneal ulcer, and hypopyon (Fig. 1) despite treatment with daily eye drops including ketorolac 5mg/mL and gatifloxacin 0.3%. Visualization by ocular ultrasound disclosed extensive involvement of anterior vitreous and lens. Cultures of corneal biopsy scrapings were performed. Samples were inoculated onto Sabouraud dextrose agar plates and incubated for 7 days at 25°C. Macroscopically, the rapid growth cultures presented a pale yellowish pigmentation on the reserve side of the plate (Fig. 2 A), and cottony colony appearance with aerial white mycelium at the surface of the plate (Fig. 2 B). Microscopically the culture revealed septated hyphae, half-moon-shaped macroconidium, and microconidia (Fig. 2C). The fungus was identified as Fusarium spp based on morphological features. The morphological identification of Fusarium could be confirmed for multilocus sequence typing (MLST). This method is based on two-locus DNA sequence-based typing schemes, including portions of the translation elongation factor 1α (EF-1α) and the second-largest subunit of RNA polymerase (RPB2). Our isolate was identified as Fusarium solani species complex (FSSC), F. solani haplotype 5-n (NRRL 32741). Antifungal susceptibility testing was performed by broth microdilution method, CLSI document M38-A2.3 The patient was initially treated with vancomycin and ceftazidime eye drops, atropine and oral acetazolamide 250mg. In addition, the patient underwent keratoplasty with anterior chamber wash and intravitreal injection of vancomycin 10mg/mL, ceftazidime 20mg/mL and amphotericin B 0.1%. Due to the high suspicion of intraocular commitment, the patient underwent a posterior vitrectomy through with temporary keratoprosthesis implants and underwent treatment with intravenous voriconazole 200mg every 12h and amphotericin B deoxycholate 1mg/kg once a day. The fungal showed susceptibility to natamycin with MIC value of 2μg/mL, and reduced sensitivity to voriconazole and amphotericin B, with higher MIC values of 32 and 16μg/mL, respectively. Resistance was demonstrated against itraconazole and fluconazole (>64μg/mL). Considering the poor visual prognosis and the multiresistant nature of the fungus, it was decided to eviscerate the left eye.
Fusarium reverse (A) and surface colony (B) on SDA culture after 1 week of incubation at 25°C. Microscopy of F. solani lactophenol cotton-blue stain with abundant macroconidia and ellipsoidal microconidia (0–1-septate) observed by microcultivation in a 7-day old culture: Note the conidiophores and conidia (C), ×400bar 200μm.
Globally, the FSSC is the most common pathogen that causes fungal keratitis. F. solani is the predominant species (found in up to 91% of isolates) pathogenic to the eye. Most infections have been reported in rural workers, and the infections are often preceded by trauma. These data support our findings, our patient was a farmer who suffered trauma to his left eye and developed an aggressive fungal keratitis.4
Considering the lower MICs for members of the FSSC, amphotericin B and voriconazole are the antifungal agents of choice for treatment of Fusarium keratitis. However, these antifungal options were not effective for our patient.5 In fact, the in vitro antifungal test showed high MIC values to almost all antifungal agents available, except for natamycin. Unfortunately, access to this medication was not available. F. solani usually exhibit high MICs for fluconazole and itraconazole. Echinocandins are not active against Fusarium spp. Second-generation triazoles such as posaconazole appear to be promising for the treatment of fungal infections of the eye. In fact, posaconazole was shown to penetrate the vitreous humor as well as the aqueous humor in a patient with a F. solani keratitis and endophthalmitis. Many studies have been published and consensus has not been reached on the best drugs to treat Fusarium keratitis. The topical antifungal agent of choice in Fusarium keratitis is natamycin (also known as pimaricin) 5%, but delayed diagnosis may lead to an insufficient response because the penetration of natamycin through the corneal epithelium is poor. Antifungal testing and precise identification of species of Fusarium contribute to the understanding of the epidemiology and guiding treatment of this difficult-to-treat infection.
DisclosuresThe authors declare no conflict of interest.
This study was supported in part by CNPq (Brazilian Coucil of Resarch).