Myiasis is a temporary infection of the skin or other organs with fly larvae.1 The larvae develop into boil-like lesions. Creeping sensations and pain are usually described by patients. Following the maturation of the larvae, spontaneous exiting and healing is experienced. Herein we present a case of a traveler returning from Central African Republic. She does not recall insect bites. She never took off her clothing for recreational bathing, nor did she visit any rural areas. The lesions appeared on unexposed skin. The specific diagnosis was performed by morphologic characterization of the larvae, resulting in Cordylobia anthropophaga, the dominant form of myiasis in Africa. To our knowledge, this is the first reported case of C. anthropophaga in Latin America.
Myiasis is a parasitic disease caused by the larval stages of dipterous flies.1 It is classified according to the infection site in cutaneous, urogenital, oral, ocular, and nasal. Cutaneous disease is the most common form,2 which is subdivided into three possible presentations based on clinical features and causative genus: furuncular, migrans, and wound myiasis.3Cordylobia anthropophaga cases have been reported in travelers retuning from Africa in countries such as the United States,4 United Kingdom,5 Korea,6 and Portugal.7 Usually, C. anthropophaga produce multiple, subcutaneous, actively growing furunculous lesions, whereas Dermatobia hominis, in humans, produce only one.8 To our knowledge this is the first reported case of Cordylobia spp. in Latin America.
Case presentationA 46-year old female presented to our clinic on September 11, with four swollen, non-painful boil-like lesions on her right lumbar and gluteal regions.
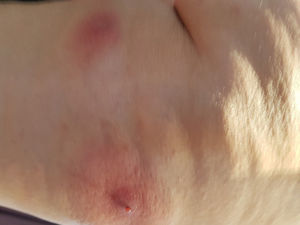
The patient had been in Bangui, Central African Republic from August 13 to August 25, 2017. While on her flight to Geneva on August 25, she started feeling a stinging sensation on her right lumbar fossa, with no other associated symptoms. She did not recall any trauma or insect bites. Upon arrival to Geneva she sought medical attention for increasing pain and erythema in two well defined, non-elevated nodular lesions on her right lumbar fossa and two other lesions of similar characteristics on her upper right gluteal region. A general practitioner prescribed an antihistamine agent and referred her to a dermatologist. Upon her visit to the dermatologist, on August 30, the lesions had become warm, tender, elevated, and suppurative. The dermatologist recommended a 7-day regimen of trimethoprim-sulfamethoxazole and analgesics. However, the symptoms did not improve. She traveled to Panama on September 2. The following night, she woke up in pain and noticed blood and a purulent discharge from the lesions (see Fig. 1), along with one oval shaped, white “worm” of approximately 1cm in length that had emerged from one of the aforementioned lesions. She visited a local emergency room the morning of September 4, where the wounds were occluded with petroleum jelly and three additional fly larvae were extracted (see Fig. 2).
Upon examination in our clinic on September 11, she had four round, erythematous, non-tender, 1.5cm in diameter, furunculous lesions with ulcerated centers on the right lumbar and upper gluteal areas. There was no evidence of bacterial superinfection.
The rest of her medical history and physical exam were non-contributory. She had no other recent foreign travel and her immediate contacts were all well. She was on no regular medication and had no known allergies.
Two days later, on September 13, she noticed a pale, non-tender nodule on her lower vermillion border on the right side of the lips. She was advised to apply petrolatum jelly to the area. This nodule experienced involution over the next four days. There was no evidence of larval presence in this lesion.
The original four lesions had resolved completely by September 18.
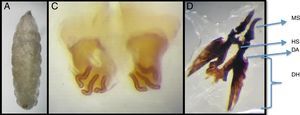
The etiologic diagnosis was performed by morphologic evaluation of the larvae (refer to Fig. 3). It was identified as C. anthropophaga. Microscopic observation of the specimens demonstrated whitish, 12 ten mm-long segment larvae, with the following features: imperceptible posterior tracheal trunks, anterior spiracles with nine branchii, posterior spiracles with three respiratory openings and no peritreme, complete spine rings in segments III to VIII, and less pronounced, non-chitinized spines in segments IX to XII.
Morphologic characterization of a Cordylobia anthropophaga larva that was extracted from one of the four lesions. (A) Third instar larvae of 12 segments, 10mm length by 2.5mm width. (B) Posterior spiracles with three curved respiratory openings without peritreme or button. (C) Cephalopharingeal skeleton: MS, mandibular sclerite strongly curved; HS, hipopharingeal sclerite; DH, dorsal horn with acute anterior process in the dorsal arc (DA). Courtesy of Magister Argentina Ying. Faculty of Medicine, Universidad de Panama.
The genera involved in human myiasis infections are D. hominis, which is the primary human botfly; Cochlomya homnivorax, or the screwworm fly in the New World; Chrysomya bezziana, or the screwworm fly in the Old World; and C. anthropophaga. Some other flies in the genera Cuterebra, Oestrus and Wohlfahrtia are animal parasites that occasionally infect humans.3 The two most common species affecting humans are D. hominis or the bot fly and C. anthropophaga9 or the tumbu fly. Transmission of D. hominis larvae is indirect: they lay eggs on the surface of hematophagous arthropods, an interaction known as “phoresia”. When the carrier arthropod reach man or animals, larvae leave eggs and penetrate the skin. Literature states that the dermatosis in Cordylobia spp. occurs via biophagous intermediate larvae, since they oviposit over damp clothing; and once hatched, 2–4 days later penetrate fabric and skin without being noticed by the host. Larvae exit the skin after 8–12 days.8 Furuncular myiasis is characterized by painful nodules in the skin with circumscribed inflammation of the dermis and subcutaneous tissue, enclosing a central slough by which the mature larvae will emerge. Multiple lesions, each containing a single larva may be present, especially with the tumbu fly.10
The patients do not usually recall an insect bite, but do notice a papule that slowly enlarges to become a painful 1–3cm sized nodule.11 They also often describe a crawling or creeping sensation. The pain is correlated with the parasites’ activity and lytic reaction.11 Scarce serosanguinous fluid is consistently reported to drain from the lesions.5,6,12 Our patient described a bloody and pus like discharge that stained her bed sheets. This secretion includes larval excretions. Treatment includes the occlusion of the lesions with dense ointments, such as petroleum jelly, to provoke hypoxia and the removal of the undamaged larvae. In rare occasions, surgical debridement is necessary. After the spontaneous exit of the first larva, our patient had petrolatum jelly applied to the three occupied lesions and three remaining larvae were extracted in the emergency department.
Patients usually do not remember contact with adult flies. This is consistent with the fact that adult flies are non-parasitic and do not directly deposit eggs on human skin, in contrast with D. Hominis that do oviposit directly on skin. Zump, in 1965 described the adult flies as stout and compact, averaging about 9.5mm in length, light brown in color, with diffuse blue-gray patches on the thorax and dark gray on the posterior part of the abdomen, with yellow face and legs.13
The adult female lays around 300–500 eggs in a lifespan and does so in two separate batches of about 100–300. Ova are preferably deposited in wet sandy soils12 or damped clothing,4 after which they hatch in 24–72h. The first larval stage, or L1, could survive without nourishment for about nine days. The L1, from wet soil or fabric, attaches to skin by means of mandibular hooks. It is described that thinner skins are favored, such as those of children; nonetheless it affects all age groups. Two to four days pass before the second larval stage, L2, develops, and another one to three days before the third larval stage, L3, is formed. The fully mature instar, L3, naturally emerges off the skin at about 9–12 days,4 then it falls on the ground and pupates after another 12 days in tropical temperatures12 or up to 26 days at lower temperatures.14 In our patient, the time lapse between the onset of symptoms and the expulsion of the L3 was 10 days. Some important differences of C. anthropophaga when compared with D. hominis are summarized in Table 1:
Clinical presentation and infective characteristics of Cordylobia anthropophaga in contrast with Dermatobia hominis.
| Cordylobia anthropophaga | Dermatobia hominis | |
|---|---|---|
| Eggs carried by other arthropod15 | No | Yes |
| Time of hatching16 | Already hatched L1 when contact occurs | L1 is hatched once contact with human body occurs, due to the temperature stimulus |
| Potential time cohabitating with humans16 | Eight to 12 days | Five to 12 weeks |
| Ability of host to recall a possible contact16 | Almost always | Occasionally |
| Number of lesions15 | Multiple | Singular |
| Anatomic location of lesions15 | Unexposed skin | Exposed skin |
Prevention strategies for travelers include drying their clothes hanging instead of placing them over a flat surface, ironing them before wearing, because heat is hypothesized to kill the larvae,17 and avoid being barefoot.
Even though our patient resides in an endemic country for D. hominis, she had been in Sub-Saharan Africa, and hence, suspicion for C. anthropophaga should have been raised from the beginning. Given the fact that Cordylobiasis has never been reported in our region, travel history and awareness of its existence were key elements in the diagnostic approach. To confirm the diagnosis, morphologic identification should always be carried out by an experienced entomologist.