Development of drug-resistance mutations is the main cause of failure in antiretroviral therapy. In Brazil, there is scarce information on resistance pattern for patients failing antiretroviral therapy.
ObjectivesTo define the HIV mutational profile associated with drug resistance in Brazilian patients from 5 large cities, after first, second or further failures to antiretroviral therapy.
MethodsWe reviewed genotyping results of 1520 patients failing therapy in five Brazilian cities. Frequency of mutations, mean number of active drugs, viral susceptibility to each antiretrovirals drug, and regional differences were assessed.
ResultsMean time of antiretrovirals use was 22.7±41.1 months. Mean pre-genotyping viral load was 4.2±0.8log (2.1±2.0 after switching antiretrovirals). Mean number of remaining active drugs was 9.4, 9.0, and 7.9 after 1st, 2nd, and 3rd failure, respectively. We detected regional variations in drug susceptibility: while BA and RS showed the highest (∼40%) resistance level to ATV/r, FPV/r and LPV/r, in the remaining cities it was around half of this rate. We detected 90% efavirenz/nevirapine resistance in SP, only 45% in RS, and levels between 25% and 30% in the other cities. Regarding NRTI, we found a similar pattern, with RJ presenting the highest, and CE the lowest susceptibility rates for all NRTI. Zidovudine resistance was detected in only 3% of patients in RJ, against 45–65% in the other cities. RJ and RS showed 3% resistance to tenofovir, while in CE it reached 55%. DRV/r (89–97%) and etravirine (61–85%) were the most active drugs, but again, with a wide variation across cities.
ConclusionsThe resistance mutational profile of Brazilian patients failing antiretroviral therapy is quite variable, depending on the city where patients were tested. This variation likely reflects distinctive choice of antiretrovirals drugs to initiate therapy, adherence to specific drugs, or circulating HIV-1 strains. Overall, etravirine and DRV/r remain as the most active drugs.
Resistance to antiretrovirals (ARV) is a usual finding in HIV-infected patients failing antiretroviral therapy (ART). The mutational pattern after initial failure is quite predictable, but in subsequent ARV regimens it may become very complex, and frequently limit the available treatment options.1 In addition, the use of different algorithms for interpretation of mutational profiles obtained by genotypic tests might provide divergent results regarding sensitivity of HIV to ARV drugs to be used in salvage therapy regimens.
The Brazilian Ministry of Health (BMOH) provides free universal access to ARV drugs since the beginning of the epidemic. The current official recommendation for patients failing therapy is to choose salvage regimens according to HIV-1 antiretroviral drug sensitivity, assessed by genotypic resistance tests.2 However, although resistance tests are supposed to be readily available, many logistical problems have impaired this strategy, due to long turnaround time of results in some areas of the country, and to the fact that many physicians decide to switch therapy without a previous resistance test.
In addition, the genotypic characteristics of HIV in patients failing ART is still unclear, since the available information is restricted to small, specific groups of patients, from different sites. In Brazil, circulation of different viral subtypes has been reported, with variable prevalence according to different regions.3–6 There is scarce information on regional differences regarding mutational profile, availability of remaining active drugs, and the variability of susceptibility rates for different ARV drugs, according to the use of different algorithms.
In the last years, the routine tool for genotypic interpretation was a locally developed algorithm (RENAGENO), in which interpretation of results are released along with that provided by TruGene platform (Siemens Healthcare Diagnostics, Inc, USA).7 In last decade BMOH trained around 400 doctors for interpreting genotypic reports, and to provide suggestions for the next ARV regimen to be used by physicians. This strategy made easier the selection of appropriate ARV drugs in salvage therapy regimens.
In the present work we evaluated a large number of resistance tests, in five large Brazilian cities. This allowed us to define the frequency of mutations after first or subsequent failures, as well as the differences between drug susceptibility rates across these locations, and mean number of remaining active drugs for patients at each site.
MethodsWe reviewed reports of resistance tests performed from 2010 to 2013 in five large Brazilian cities, from different regions. All available reports from patients tested in reference centers for HIV care in Porto Alegre (RS, South region), Campinas (SP, Southeast region), Rio de Janeiro (RJ, Southeast region), Vitoria (ES, Southeast region), Salvador (BA, Northeast region), and Fortaleza (CE, Northeast region) were reviewed.
Frequency of detected drug-resistance mutations (DRM), previous use of antiretroviral drugs, and patients’ characteristics were evaluated, and the mean number of fully active drugs was calculated for each city and for the overall study population. Fully active drugs were attributed a weight equal to one. Drugs partially active (“intermediate resistance”) were given a 0.5 weight, while for complete resistance the attributed weight was zero. The susceptibility for each drug was compared by using the Brazilian algorithm of interpretation (RENAGENO), according to patient's origin.7
Statistical analysesWe used SPSS (Statistical Package for Social Sciences) version 17.0 to perform all statistical calculations. Descriptive analyses (frequencies, mean, standard deviations) were performed, and comparisons of frequencies between groups were assessed by chi-square test.
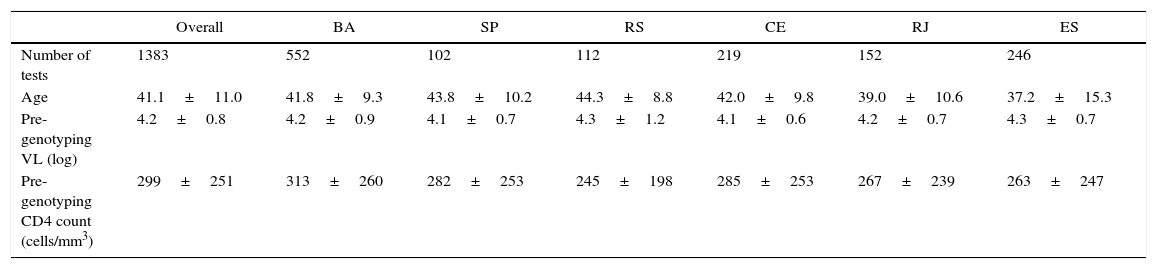
ResultsA total of 1512 genotypic tests was reviewed in the study period, but only 1481 had enough information to be included in the analysis. Most tests were performed in patients from Salvador (552), followed by Vitória (246), Fortaleza (219), Rio de Janeiro (152), Porto Alegre (112), and Campinas (102 tests). Table 1 shows the main characteristics of patients submitted to HIV-1 genotyping.
Characteristics of patients submitted to HIV-1 genotyping in five different cities in Brazil from 2010 to 2013.
| Overall | BA | SP | RS | CE | RJ | ES | |
|---|---|---|---|---|---|---|---|
| Number of tests | 1383 | 552 | 102 | 112 | 219 | 152 | 246 |
| Age | 41.1±11.0 | 41.8±9.3 | 43.8±10.2 | 44.3±8.8 | 42.0±9.8 | 39.0±10.6 | 37.2±15.3 |
| Pre-genotyping VL (log) | 4.2±0.8 | 4.2±0.9 | 4.1±0.7 | 4.3±1.2 | 4.1±0.6 | 4.2±0.7 | 4.3±0.7 |
| Pre-genotyping CD4 count (cells/mm3) | 299±251 | 313±260 | 282±253 | 245±198 | 285±253 | 267±239 | 263±247 |
All results are expressed as mean±standard deviation.
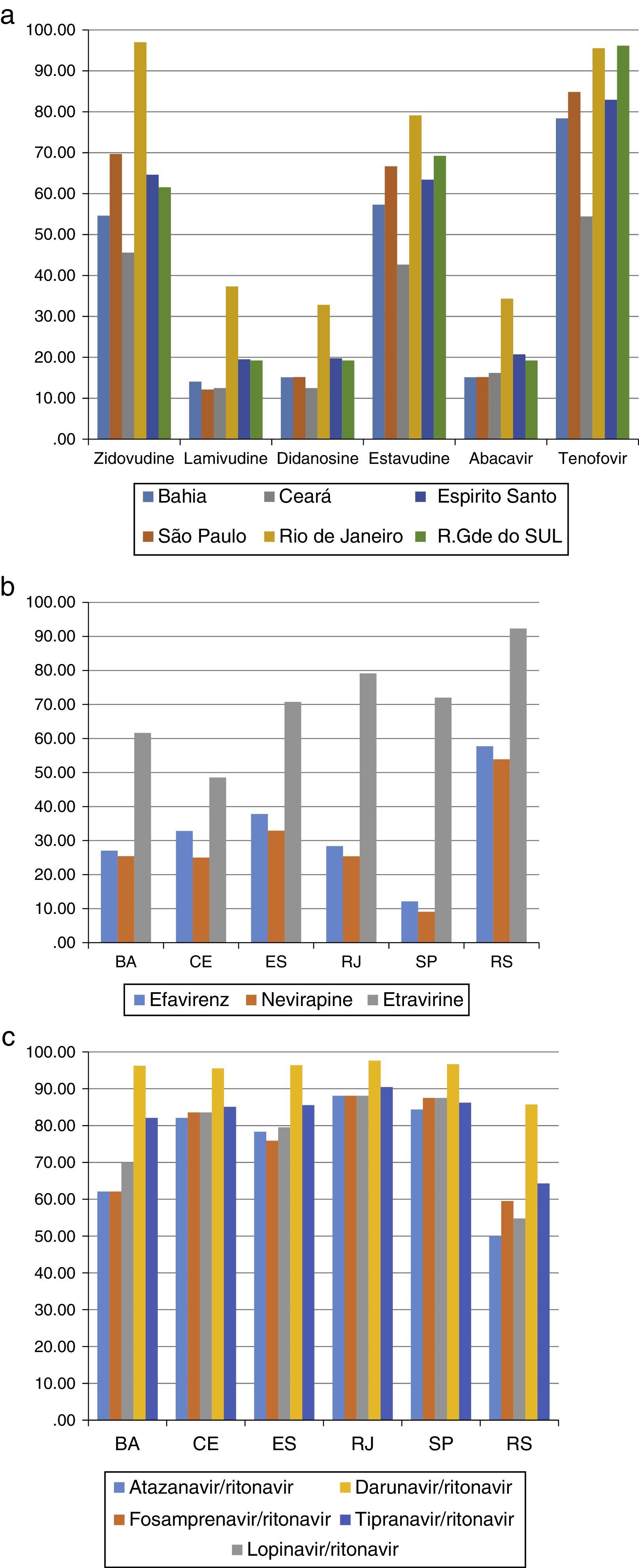
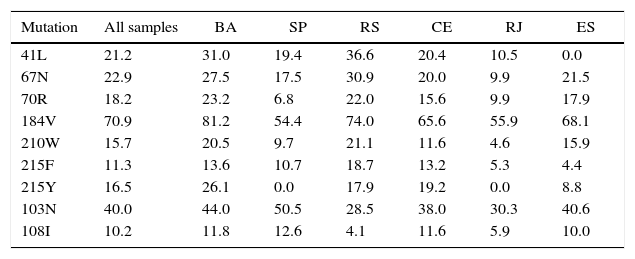
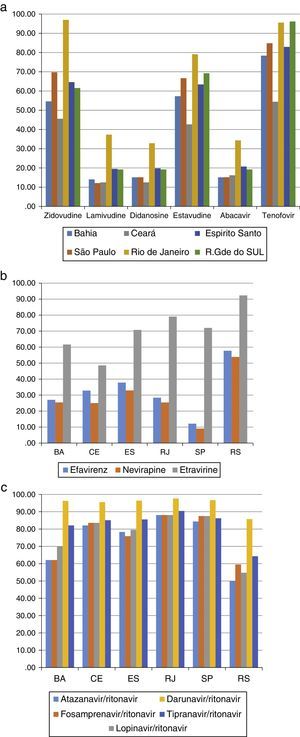
Most patients (675 subjects – 43.1%) were failing their second ARV regimen, 431 (37.8%) were failing their first ARV treatment, and the remaining 375 (15%) had already failed three or more regimens. The most frequently detected DRM (>10%) are shown in Tables 2 and 3. The susceptibility rates of HIV-1 strains to each ARV drug in the five different locations are shown in Figure 1a–c. It should be pointed out the clear difference between the susceptibility rates to reverse transcriptase inhibitors (zidovudine – AZT, tenofovir – TDF, didanosine – ddI, stavudine – d4T, nevirapine – NVP, efavirenz – EFV, and etravirine – ETV) among tests originated from different sites (p<0.001 for all comparisons). For instance, genotypic tests of patients from SP showed the highest rate of resistance to EFV and NVP (92%), while in RS almost 2/3 (58%) of tests detected susceptibility to these non-nucleoside reverse transcriptase inhibitors (NNRTI, p<0.001). The remaining three sites shared a similar profile, with detected susceptibility for NNRTI in around 1/3 of samples. We observed the same pattern for all drugs, with a large variability in susceptibility rates for different cities, especially for some ARV: in CE we detected resistance to ETR in 52% of samples, while in RS only 8% of tests showed resistance to this drug (p<0.001).
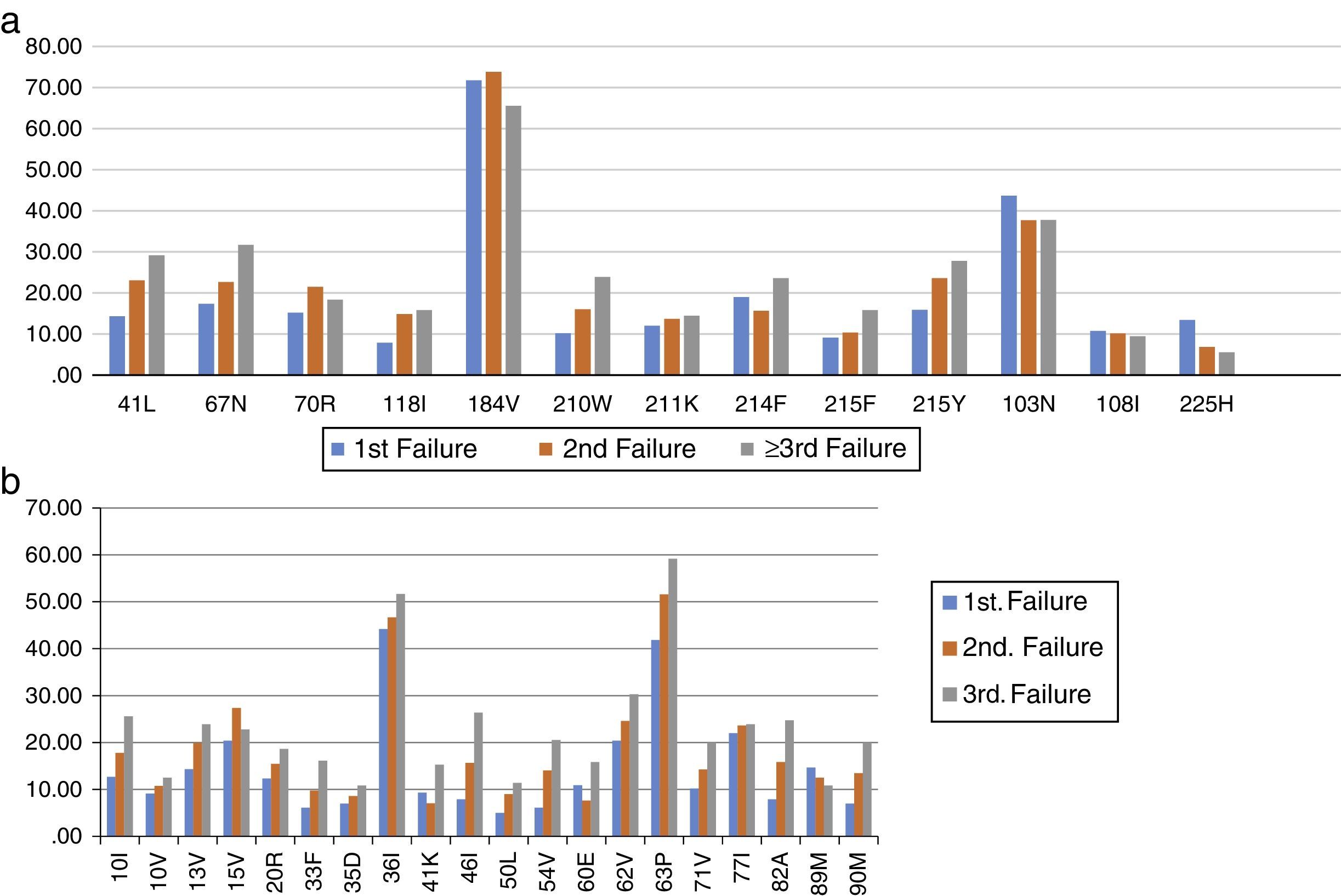
Frequency (%) of most common drug-resistance mutations to HIV-1 reverse transcriptase inhibitors, according to the patient's origin.
| Mutation | All samples | BA | SP | RS | CE | RJ | ES |
|---|---|---|---|---|---|---|---|
| 41L | 21.2 | 31.0 | 19.4 | 36.6 | 20.4 | 10.5 | 0.0 |
| 67N | 22.9 | 27.5 | 17.5 | 30.9 | 20.0 | 9.9 | 21.5 |
| 70R | 18.2 | 23.2 | 6.8 | 22.0 | 15.6 | 9.9 | 17.9 |
| 184V | 70.9 | 81.2 | 54.4 | 74.0 | 65.6 | 55.9 | 68.1 |
| 210W | 15.7 | 20.5 | 9.7 | 21.1 | 11.6 | 4.6 | 15.9 |
| 215F | 11.3 | 13.6 | 10.7 | 18.7 | 13.2 | 5.3 | 4.4 |
| 215Y | 16.5 | 26.1 | 0.0 | 17.9 | 19.2 | 0.0 | 8.8 |
| 103N | 40.0 | 44.0 | 50.5 | 28.5 | 38.0 | 30.3 | 40.6 |
| 108I | 10.2 | 11.8 | 12.6 | 4.1 | 11.6 | 5.9 | 10.0 |
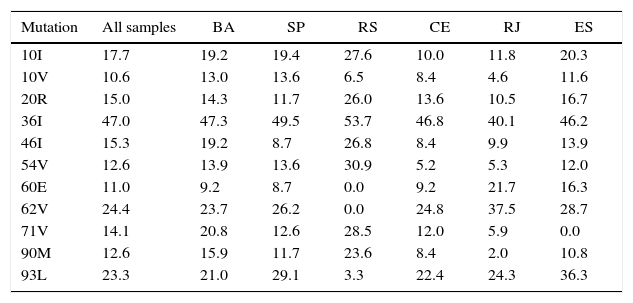
Most frequent (>10%) drug-resistance mutations in HIV-1 protease, according to origin of sample.
| Mutation | All samples | BA | SP | RS | CE | RJ | ES |
|---|---|---|---|---|---|---|---|
| 10I | 17.7 | 19.2 | 19.4 | 27.6 | 10.0 | 11.8 | 20.3 |
| 10V | 10.6 | 13.0 | 13.6 | 6.5 | 8.4 | 4.6 | 11.6 |
| 20R | 15.0 | 14.3 | 11.7 | 26.0 | 13.6 | 10.5 | 16.7 |
| 36I | 47.0 | 47.3 | 49.5 | 53.7 | 46.8 | 40.1 | 46.2 |
| 46I | 15.3 | 19.2 | 8.7 | 26.8 | 8.4 | 9.9 | 13.9 |
| 54V | 12.6 | 13.9 | 13.6 | 30.9 | 5.2 | 5.3 | 12.0 |
| 60E | 11.0 | 9.2 | 8.7 | 0.0 | 9.2 | 21.7 | 16.3 |
| 62V | 24.4 | 23.7 | 26.2 | 0.0 | 24.8 | 37.5 | 28.7 |
| 71V | 14.1 | 20.8 | 12.6 | 28.5 | 12.0 | 5.9 | 0.0 |
| 90M | 12.6 | 15.9 | 11.7 | 23.6 | 8.4 | 2.0 | 10.8 |
| 93L | 23.3 | 21.0 | 29.1 | 3.3 | 22.4 | 24.3 | 36.3 |
(a) Susceptibility of HIV-1 (%) to NRTI drugs after 1st treatment failure in five cities of different Brazilian states. (b) Susceptibility of HIV-1 to NNRTI after 1st treatment failure, according to patient's origin. (C) Susceptibility (%) of HIV-1 to PIs after 1st treatment failure, according to patient's origin.
The resistance rates to protease inhibitors (PI) also significantly varied according to origin of patients. Patients tested in BA or RS showed the highest (30–50%) resistance rates to boosted atazanavir (ATV/r), fosamprenavir (FPV/r), and lopinavir (LPV/r) while those living in the remaining cities had levels of resistance to this class below 20%, as shown in Figure 1c (p<0.001). Of note, resistance to boosted-darunavir (DRV/r) was detected in less than 5% of samples, except in RS, where we found 14% of samples presenting resistance to that PI. DRV/r was the only ARV drug that preserved susceptibility regardless of patient's origin.
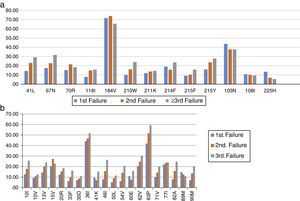
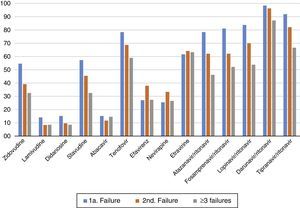
The most frequent drug-resistance mutations after first, second, and third failures are shown in Figure 2. The overall mean number of active drugs was 8.8±3.2, 9.2±2.7, and 7.9±3.9 for patients failing first, second, or third/more ARV regimens, respectively. This number was similar for the different cities, and ranged from eight (BA) to 10.5 (RJ). Some specific mutations like 65R also showed a great variation, with an overall mean prevalence of 4.4%, but ranging from 2.4% in RS, to 6.2% in RJ (p<0.01).
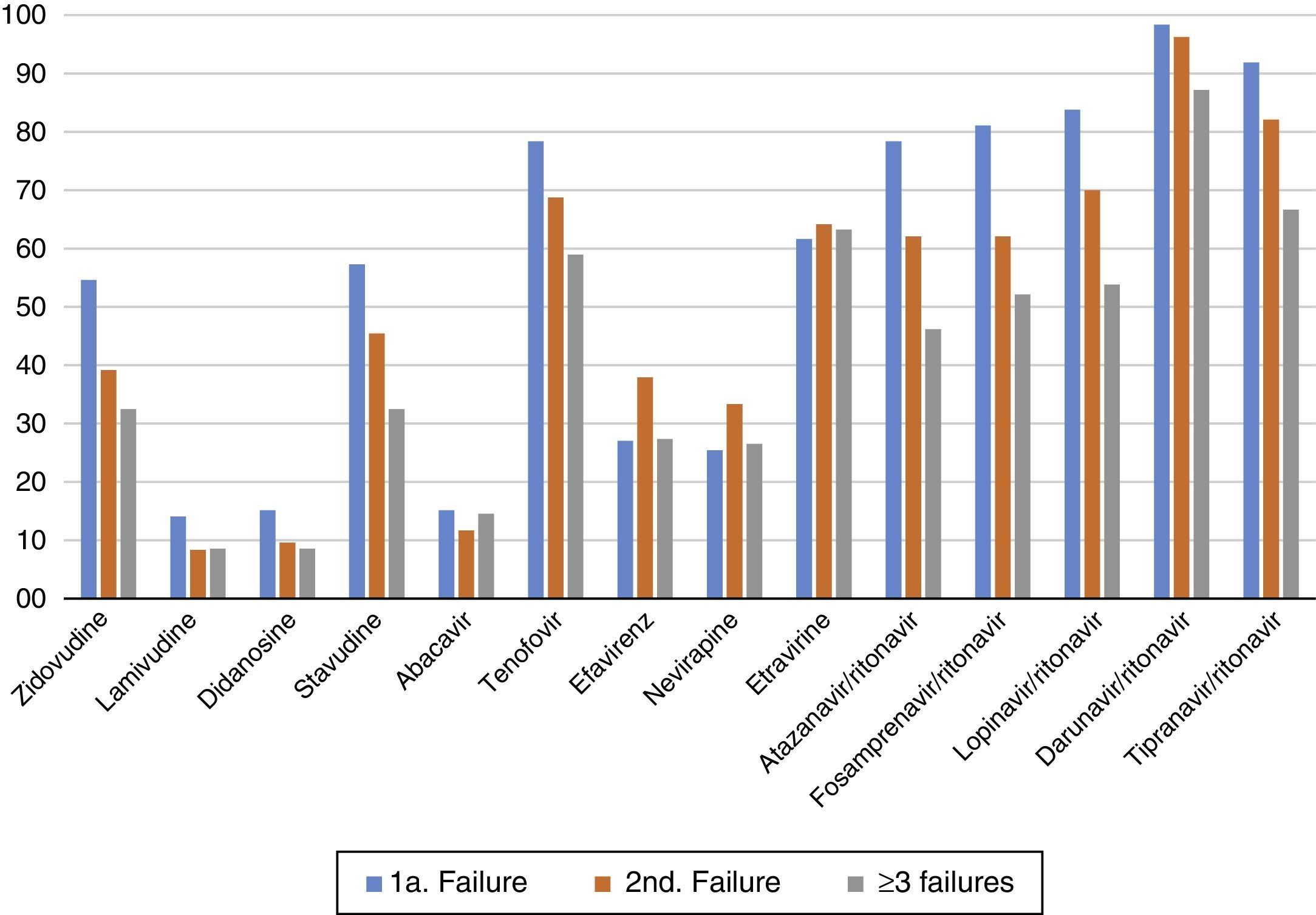
The susceptibility pattern for all ARV drugs decreased over subsequent failures, except for ETR and DRV/r which kept the same level of activity against HIV-1 across initial or subsequent ARV regimens failures, as summarized in Figure 3.
The frequency of HIV-1 subtypes was also variable across cities. As expected, most strains were classified as subtype B. The lowest (55.4%) and highest (78.6%) subtype B frequency was observed in ES and BA, respectively. Subtype C was mainly detected in RS (31.7%), with other sites presenting a much lower frequency (0.4–3.8%). Subtype F was less frequently detected in RS (3.2%), with the highest frequencies observed in CE (9.6%) and ES (11.5%). Recombinant BF strains were mostly detected in BA and SP (4.7% each), but absent in CE. Other multiple recombinant forms were also detected in all sites, ranging from 3.5% in RS to 29.9% in ES (p<0.01 for comparisons between sites).
DiscussionWe detected a high variability in susceptibility rates to most ARV drugs, according to patient's origin. The only ARV drug with a stable susceptibility rate, regardless of location, was DRV/r. The most marked differences were found in genotypic tests from RS, RJ, and CE, which showed a distinct pattern of susceptibility to NRTI when compared to BA or ES. We observed that susceptibility rates to these drugs were significantly higher in RJ in comparison to other locations, while for ddI and TDF the rates in RJ were different from BA, CE, and ES, although similar to that found in RS.
Noteworthy, we detected a high susceptibility rate (97%) for AZT in RJ, in contrast to CE, where only 55% of samples were susceptible to AZT. This may reflect the preferential use of AZT or TDF as first line therapy for the remaining sites, where we observed a contrasting susceptibility between TDF and AZT, but it was not detected in RJ, where both drugs tested active for almost 100% of samples. A potential explanation for this fact could rely on distinct adherence rates to first line therapy in different sites.
For NNRTI drugs the pattern was also different, with RS showing an almost two-fold higher (>50%) susceptibility rates to EFV and NVP, in comparison with other sites (25–35%, p<0.001 for comparison with other sites). In contrast, SP showed the lowest susceptibility rates (around 10%) for these drugs. Even for ETR, a drug not used as first-line therapy, we detected significant differences between sites, with lower susceptibility rates found in CE (48%), and higher rates in RS (92%) and RJ (79%). For PI, we found a more homogenous picture, with CE, ES, RJ, and SP showing similar (around 80%) susceptibility rates for all drugs in this class. However, in RS and BA we found much lower susceptibility rates for ATV/r, LPV/r, and FPV/r.
Several previous studies have shown a similar level of drug resistance in different Brazilian regions; however the sample size were usually smaller than ours, and most analyses were restricted to a specific setting.8–13 Even in such populations we can notice different prevalence of mutations like 103N. Toledo et al. evaluated a sample of 467 genotypic tests in Parana state, and detected a 46% prevalence of 103N mutation, while Westin et al., in Minas Gerais state found a lower prevalence value (32%).10,5 In Northeast Brazil, Cavalcanti et al. reported 58.7% resistance to NNRTI, with 103N and 190A as the most prevalent drug resistance mutations in this class.13
We did not get information on most used first-line ARV regimens in each city, but the Guidelines of the BMOH recommends to use as initial ART regimens two NRTI drugs plus EFV or NVP.2 Our results suggest that compliance with these recommendations are distinct in different settings. The distinct susceptibility rates detected suggest that in some settings (BA and RS) protease inhibitors were more frequently used as the third drug either in first-line or salvage regimens, while in others NNRTI were the drug of choice for starting therapy. Other alternative explanation is a wider previous use of unboosted PI (nelfinavir, indinavir, or atazanavir), since mutations like 90M and 36I, and 71V are associated with use of these drugs.
This possibility is supported by the finding of an inverse relationship between susceptibility rates for PI, compared to NNRTI drugs in the most divergent scenario: the lowest susceptibility rates for PI (especially ATV/r and FPV/r) and the highest rates for NNRTI observed in RS. However, this was not true for SP, where we detected the lowest susceptibility rates for NNRTI, but no difference for PI. For ETR, the trend was similar, except for CE, where we detected 48% susceptibility, against higher rates (62%-92%) in the remaining sites. Interestingly, all ITRN showed better activity in RJ, in comparison to other cities, but NNRTI susceptibility rates were similar across sites.
We know that previous use of NVP or EFV does not imply in cross-resistance to ETR.14,15 However, if patients remain on failing regimens containing these drugs for long enough time, it is expected that viral variants with a more complex DRM profile will eventually be selected, which could lead to cross-resistance to ETR. Again, the lack of information on how long these patients remained on failing regimens limits the reach of such conclusions.
One interesting point is the finding that patients still preserve a high GSS, even considering that most had previous treatment failure to more than one ARV regimen. Therefore most failing patients can be successfully treated, with a high likelihood to achieve sustained viral suppression. This is in accordance to recent publications by Brazilian authors showing that even heavily pre-treated patients are able to reach virological suppression after one year of a salvage regimen including new ARV drugs/classes.16–19 On the other hand, low resistance rates after failing regimens suggests that adherence to therapy is a major component of treatment failure in these centers, and it deserves a careful evaluation by physicians and health authorities.
The observed frequency of subtypes also demonstrates the different epidemiological scenario in the distinct regions. While most HIV strains belonged to subtype B, a clear divergence regarding subtype C was observed, which was basically restricted to RS, but was also present in SP, BA, and ES, as already detected in previous studies.20,21 Noteworthy, ES showed the lowest frequency of subtype B (55.4%), but the highest proportion of other recombinants (29.9%), suggesting a distinct dynamic of HIV-1 diversity in that area. Whether subtypes variation could explain some differences in mutational profiles observed in different cities, it is still a controversial issue.22–26
Although mutations associated with HIV-1 subtype C (like V106N) may confer resistance to EFV and NVP they have no impact on ETV activity.27 In addition, in the present study it was not a frequent finding. Some recent data indicate that subtype C-associated mutations conferring resistance to ETV are rare, and most HIV-1 strains are susceptible to these drugs after failure to first generation NNRTI.28 The higher susceptibility rate to ETV detected in RS (the site presenting the higher frequency of subtype C) underscores the absence of an association between HIV-1 subtype and susceptibility rate to ETV.
Our study has some clear limitations: we did not have access to information on treatment outcomes after therapy modification, which precluded confirmation that the observed susceptibility rates held true in clinical practice. In addition, information on adherence to therapy was not available, which could explain some of study findings.
However, this study has a large sample size, and includes patients from different Brazilian regions, providing information on the most frequent DRM, and susceptibility rates for patients in different phases of treatment of HIV infection. In addition, it underscores the need for virological monitoring to detect drug failure, and to use genotypic tests to guide the choice of salvage therapy regimens, as already suggested in other studies on drug resistance in resource-limited settings.29 Understanding the factors associated with failing treatment, and the distinct resistance profile in early or later treatment failures is an essential step to face this persistent challenge in HIV therapy.
Conflicts of interestThe authors declare no conflicts of interest.
This work was supported by an Investigator's Initiative Grant, funded by Janssen-Cilag Farmacêutica Ltda.