Inappropriate use of antibiotics leads to increased levels of bacterial resistance making it difficult to treat upper respiratory tract infections. The appropriate use of these drugs has a fundamental role in controlling resistance and in success of treatment of childhood infections. Therefore, the aim of this study was to assess the prescription and use of antibiotics for Brazilian children.
MethodsThe use of antibiotics in 160 children was monitored in two Primary Health Centers by questionnaires administered to parents and caregivers that assessed the social, demographic and clinical conditions of the children. Furthermore, the antibiotic use pattern was ascertained in these children and compared to the recommendations of the Brazilian and international guidelines.
ResultsThe use of these drugs had an inverse relationship with children breast-fed to six months of age, showing that breast-fed children had a tendency to use less of these drugs. There was great variability in the amoxicillin doses used for upper respiratory infections ranging from 8.2 to 91.9mg/kg/day. The doses used in most treatments were far below the doses recommended in the Brazilian and international guidelines (50% and 97%, respectively).
ConclusionAlthough there are guidelines for the use of these medications, compliance is still very low, leading to under dosage and therapeutic failures. It is essential for pediatricians to be aware of and comply with the guidelines, avoid personal decisions and take measures based on strong clinical evidence. The proper use of these medications, in addition to greater therapeutic success, decreases the possibility of the appearance of resistant microorganisms.
Antibiotics prescription for children has been a cause of concern throughout the world. Their excessive use in non-established infections or in infections with viral etiology has been the concern of many authors.1–4
The use of these drugs in infections with viral etiology is not only ineffective but has also served as a form of selective pressure for the appearance of increasingly resistant microorganisms. As an example, Streptococcus pneumoniae, the main cause of upper respiratory tract infections, has increased the level of resistance to penicillins by 20–33%.5,6
Creech et al.7 showed that the presence of methicillin resistant Staphylococcus aureus (MRSA) in nasal cavities of children increased from 0.8% to 9% from 2001–2005. The reasons for the increase in bacterial resistance levels are closely linked to the improper use of antibiotics. The greatest reasons for inappropriate use of these drugs in children are pressure from the parents to prescribe antibiotics, difficulty in differentiating the etiology (bacterial or viral) of these infections and especially improper dosage as the guidelines are not followed properly.8–10
A study published in 2011 by the CDC (Centers for Disease Control and Prevention) also confirmed the direct relationship between improper antibiotic use and increase in bacteria resistance levels. Antibiotic consumption in a population of 18 million people was studied for eight years. Despite a decrease in the mean number of antibiotic prescriptions, antibiotics consumption was directly related to increased levels of resistance to S. pneumoniae and the greater the consumption, the greater the level of resistance found.5
To tackle bacterial resistance, the World Health Organization proposed, among numerous interventions, that prescribers adopt and use protocols or guidelines based on strong evidence for the use of antibiotics for community infections.11 Adopting these guidelines standardizes treatments, minimizes dosage mistakes, avoids individual decisions and prioritizes decisions based on clinical evidence.12 Recent studies have shown that not adopting guidelines has led to antibiotic prescription mistakes in pediatrics in terms of dose or duration.10,13
Thus the objective of the present study was to assess the use of antibiotics in children, regarding the suitability of indication and dose, and compare them with the guideline recommendations for acute respiratory infections.
Materials and methodsThis is a cross-sectional study, carried out by administering a questionnaire with closed questions to parents or caregivers of children aged 0–9 years who were prescribed antibiotics in Primary Health Centers in the municipality of Sorocaba, São Paulo state (Brazil) over 12 months. Sorocaba has a population of 596,000 inhabitants and HDI (Human Development Index) of 0.828 child mortality index of 13.5 deaths under one for every 1000 live births. To collect the information, two Primary Health Centers that attend 12% of the population of the municipality were chosen.14
The instrument sought social, demographic and clinical information of the children and their caregivers and information on the medical diagnosis and the antimicrobial therapy used. The instrument was designed based on a literature review of studies on the use of medications, and was previously tested and validated.10,15
Parents or caregivers of children aged 0–9 years who received prescriptions containing antibiotics at Primary Health Centers and agreed to participate in the study were interviewed.
The data were collected over 12 months and included results from 160 children. The study was approved by the Committee for Ethics in Research at the University of Sorocaba (# 003/06). Anthropometric assessment was carried out with measurements of weight and height to assess the use mg/kg/day of the antibiotics used.
The prescriptions of antimicrobial medications used were compared with the guidelines of the Brazilian Society of Pediatrics16 and the American Academy of Pediatrics.17–19
Statistical analysis was performed using the software Bioestat 5.3. The social and demographic data were treated descriptively in frequency tables. Analysis of variance (ANOVA) and Fisher's exact test were used, and the level of significance was set at 5%.
ResultsThe average age of children enrolled in this study was 45.6 months, 49.7±30.6 months for females and 41.4±28.6 months for males (Z-test, p=0.082) (Table 1).
Social and demographic characteristics of the children using antibiotics.
| Variable | n=160 | % |
|---|---|---|
| Gender | ||
| Female | 81 | 50.63 |
| Male | 79 | 49.38 |
| Age | ||
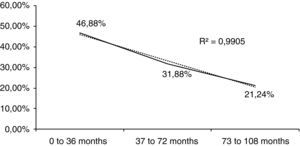
| 0–36 months | 75 | 46.88 |
| 37–72 months | 51 | 31.88 |
| 73–108 months | 34 | 21.25 |
| Breast-feeding (up to six months) | ||
| No | 33 | 20.63 |
| Yes | 127 | 79.38 |
| Children resident in the same household | ||
| 1 | 86 | 53.75 |
| 2 | 53 | 33.13 |
| 3 | 16 | 10.00 |
| 4 | 4 | 2.50 |
| 5 | 1 | 0.63 |
| Previous antibiotic use (last six months) | ||
| Yes | 103 | 64.37 |
| No | 57 | 35.63 |
Age was segmented into three groups; the majority of the participants were in the youngest age-group of 0–36 months. Fig. 1 shows these results as an almost perfect straight line (R2=0.99).
Information about previous antibiotic use provided by the parents or caregivers is showed in Table 2. Gender, living with other children in the same household, and low-birth weight (less than 2.500g) were not associated with previous antibiotic use (Fisher's Exact test, p>0.05). Breast-feeding (up to six months) was significantly associated (Exact Fisher test, p<0.0001) with antibiotic use. Lack of breast-feeding was strongly associated to greater consumption of these medications (OR=5.27; 2.31–11.96).
Previous antibiotic use (last six months) and relationship with social and demographic characteristics studied.
| Variables | Previous antibiotic use (last six months) | RR (95% CI) | p-Value | |
|---|---|---|---|---|
| Yes n. (prevalence) | No n. (prevalence) | |||
| Gender | 0.223 | |||
| Male | 60 (75.9) | 19 (24.1) | 1 | |
| Female | 54 (66.6) | 27 (33.3) | 1.14 (0.93–1.38) | |
| Other children resident in the same household | 0.861 | |||
| No | 62 (72.1) | 24 (27.9) | 1 | |
| Yes | 52 (70.3) | 22 (29.7) | 1.02 (0.84–1.25) | |
| Low-birth weight | 0.779 | |||
| No | 95 (73.1) | 35 (26.9) | 1 | |
| Yes | 12 (70.6) | 5 (29.4) | 1.03 (0.74–1.43) | |
| Breast-feeding (up to six months) | <0.0001 | |||
| No | 22 (66.6) | 11 (33.3) | 1 | |
| Yes | 35 (27.6) | 92 (72.4) | 2.41 (1.66–3.50) | |
RR, relative risk; CI, confidence interval.
Table 3 shows the diagnoses established. Pharyngitis and tonsillitis was more prevalent among the 3–6 year-old aged group (37–72 months).
Diagnoses distribution among different age groups of children (n=160).
| Age group (months) | 0–36 | 37–72 | 73–108 | Total |
|---|---|---|---|---|
| Diagnosis | n (%) | n (%) | n (%) | (%) |
| Pharyngitis and tonsilitis | 14 (27.45) | 23 (45.10) | 14 (27.45) | 31.88% |
| Flu and viruses | 14 (66.67) | 4 (19.05) | 3 (14.29) | 13.13% |
| Undefined respiratory infections | 26 (65.00) | 8 (20.00) | 6 (15.00) | 25.00% |
| Otitis | 12 (70.59) | 4 (23.53) | 1 (5.88) | 10.63% |
| Pneumonias | 2 (40.00) | 2 (40.00) | 1 (20.00) | 3.13% |
| Sinusitis | 2 (15.38) | 6 (46.15) | 5 (38.46) | 8.13% |
| Urinary tract infections | 2 (50.00) | 2 (50.00) | 0 (0.00) | 2.50% |
| Other | 3 (33.33) | 2 (22.22) | 4 (44.44) | 5.63% |
| Total | 46.88% | 31.88% | 21.25% | 100.00% |
Table 4 shows the distribution of antibiotic classes used by children enrolled in the present study in comparison to distributions reported by Marra et al.20 and Kozyrskyj et al.21 Penicillins were the most commonly prescribed antibiotic as well as in the aforementioned studies.
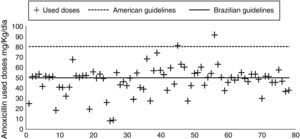
Fig. 2 shows the distribution of amoxicillin dosages used to treat upper respiratory infections (n=79) in the present study.
Table 5 shows the percentage of treatments with dosages below the recommendations of the Brazilian and American guidelines. There are a large number of treatments that did not reach 50mg/kg/day (Brazilian guideline) and almost none of the dosages reached the recommendations of the American Academy of Pediatrics (80–90mg/kg/day).
Treatments with dosage below the recommendations of the Brazilian and American guidelines.
| Diagnosis | Pharyngitis and tonsilitis | Otitis | Sinusitis | All URI |
|---|---|---|---|---|
| Dosages below 50mg/kg/day (Brazilian guidelines)18 | 51.02% | 37.50% | 61.54% | 50.00% |
| Dosages below 80mg/kg/day (American guidelines)19–21 | 97.96% | 93.75% | 100.00% | 97.44% |
URI, upper respiratory infections.
The study sample had balanced distribution of male and female children, predominantly aged less than 36 months. Other studies using the same methodology have indicated the same trend, because as age advances the immune system becomes more mature, better hygiene habits are practiced and infectious diseases are less prevalent.20–22
In addition to current use of antibiotics by the children, the assessment instrument used also inquired the parents or caregivers about antibiotic use in the last six months. The authors observed frequent and repeated use of this class of drugs. Previous antibiotic consumption was not related to gender, other children resident in the same household or low birth weight. However, absence of breast-feeding was associated with a fivefold increase in the consumption of these medications by the children.
Duration of breast-feeding and the amount of milk ingested have been related to the emergency of respiratory diseases in childhood. Chantry et al.23 stated that children breast-fed for at least six months had 1.6% likelihood of having pneumococcal pneumonia while in not breast-fed children this chance was fourfold greater–very similar data to those reported in the present study. Some studies have shown that the immunological role of maternal milk is not limited to transferred antibodies but it may also be associated to inhibition of bacterial colonization mediated by casein.24,25
The increased index of pharyngitis and tonsillitis was more frequent in 3–6 year-old children and it can be explained by the exposure of the children to the school environments, propitious for contamination and propagation of upper respiratory tract infections. Upper respiratory tract infections are streptococcal infections transmitted by physical contact between contaminated hands of sick children with the upper respiratory tract of healthy individuals. Contagion is favored in closed environments, such as day care centers and schools.26
Other data worth highlighting are the large number of respiratory diagnosis without topographic definition of the infection focus (undefined respiratory infections), representing 25% of all cases, of which 65% were in children aged 0–3 years. The difficulty in referring symptoms in this age group may have contributed to this large proportion.
About 13% of the prescriptions were written for medical diagnosis of flu and viruses, conditions where it is known to be unresponsive to antibiotics. There are plenty of studies in the literature showing the inefficacy of these drugs for these conditions as prophylactic agents.21,22,26,27 Exaggerated and uncontrolled use of antibiotics directly favors the appearance of resistant microorganisms.28
The present study assessed the use of different antibiotic classes in the treatment of upper respiratory tract infections in children. The results were compared with two similar studies. Penicillin was the most prescribed, especially amoxicillin, the drug of choice for respiratory infections in pediatrics. Similar results have been found in a recently published study.29 Although the data distribution was very similar to the studies presented, greater frequency of penicillin-containing prescriptions was clearly observed in the present study compared to other two studies (p=0.0003 for Marra et al.20 and p=0.001 for Kozyrskyj et al.21). This difference has been explained because no macrolide antibiotics were available in the municipal center so that the prescribers insisted on penicillin and greatly increased its use. In the participating Primary Health Centers, the only macrolide available was erythromycin that, although having a very similar spectrum to azithromycin has uncomfortable dosage as it requires to be administered four times daily to children under the age of ten. The possibility of prescribing azithromycin in those centers would offer a more convenient option for physicians, and greater comfort for the parents.30 The literature reports that repeating the same drugs for recurrent upper respiratory tract infections is determinant in selecting resistant microorganisms, which can be avoided by using other therapeutic options.31–33
Regarding the therapeutics used to treat upper respiratory infections (pharyngitis, tonsillitis, otitis and sinusitis), which accounted for 50.65% of the participants of the present study, the Brazilian literature has established amoxicillin at the dose of 50mg/kg/day as the preferred regimen for children without complications. For relapses and comorbidities, the dose should be increased to 90mg/kg/day.16 In contrast, guidelines from the American Academy of Pediatrics has recommended, especially for sinusitis and otitis, starting treatment with high dose amoxicillin at 90mg/kg/day because of the many reports of S. pneumoniae resistant to conventional treatment.17–19
In the present study, the computed amoxicillin doses to treat upper respiratory infections were calculated based on the prescription and weight of the children at the time of the consultation. On average, the dose used was 47.2±13.7mg/kg/day for all upper respiratory infections. For sinusitis, the average doses were 46.1±13.7mg/kg/day, for otitis 53.2±11.8mg/kg/day, and for pharyngitis and tonsillitis 45.5±15.1mg/kg/day.
There was great disparity in treatments used with dosages ranging from 8.2 to 91.2mg/kg/day. When compared to the Brazilian guideline,16 39 doses (50.0%) were below the recommended standards (50mg/kg/day) and 20 prescriptions (25.64%) did not even reach 40mg/kg/day. According to the guidelines of the American Academy of Pediatrics17–19 only two treatments (2.6%) of the present study have reached the recommended dosages (80 and 90mg/kg/day).
Sub-inhibitory amoxicillin concentrations at the infection site has led to bacterial adaptive phenomena, known as persistence, that first inhibit bacterial growth without eradicating the infection. In addition to the direct effect on the therapeutic failure, studies have shown that sub-inhibitory concentrations induce mutation phenomena, induction and expression of bacterial resistance promoting genes. Studies have shown that these genes, in addition to resistance to the drug itself, further promote cross resistance, that is, to amoxicillin and other beta-lactam antibiotics.34,35
The various percentage of treatments with dosages below those recommended by the Brazilian and American guidelines were described. There is a large number of treatments that do not reach 50mg/kg/day (Brazilian guideline)16 and almost none of the dosages reached those recommended by the American Academy of Pediatrics (80–90mg/kg/day).17–19
This study detected a great number of unnecessary prescriptions that lead to increased antibiotic exposure to non-pathogenic microorganisms. This exposure favors the appearance of bacterial resistance genes and had exposed these children to adverse effects caused by antibiotics, such as hypersensitive reactions that are very common with the use of beta-lactam antibiotics.
Another important result of the present study was the lack of standardization in antibiotic prescriptions. Even considering the Brazilian guideline, on average, 60% of the prescriptions had dosages below those recommended and showed lack of standardization in the prescriptions. The Brazilian government has produced and made available reference material for rational medication use, based on strong clinical evidence36 in addition to innumerable pediatric and infectious diseases societies that make their guidelines available on their websites. Thus prescribers need to consult and use these guides, bringing economy to the health care network and better clinical results in tackling infections in pediatrics.37–39
ConclusionIn the present study many pediatric prescriptions were found with errors regarding the indication and dosage of antimicrobials. In spite of many publications warning about the undesirable consequences of antibiotics use in undefined situations or undefined viral etiology, this study found a great number of unnecessary prescriptions, leading to increased exposure of antibiotic in cases of nonpathogenic microorganisms. This conduct favors the appearance of bacterial resistance genes in addition to exposing these children to the antibiotic adverse effects, such as hypersensitive reactions that is very common with the use of these antibiotics.
Conflict of interestAll authors declare to have no conflict of interest.