The introduction of highly active antiretroviral therapy during the 1990s was crucial to the decline in the rates of morbidity and death related to the acquired immunodeficiency syndrome (AIDS) and turned human immunodeficiency virus (HIV) infection into a chronic condition. Consequently, the HIV/AIDS population is becoming older. The aim of this study was to describe the immunological, clinical and comorbidity profile of an urban cohort of patients with HIV/AIDS followed up at Instituto de Pesquisa Clinica Evandro Chagas, Oswaldo Cruz Foundation in Rio de Janeiro, Brazil. Retrospective data from 2307 patients during January 1st, 2008 and December 31st, 2008 were collected. For continuous variables, Cuzick's non-parametric test was used. For categorical variables, the Cochran–Armitage non-parametric test for tendency was used. For all tests, the threshold for statistical significance was set at 5%. In 2008, 1023 (44.3%), 823 (35.7%), 352 (15.3%) and 109 (4.7%) were aged 18–39, 40–49, 50–59 and ≥60 years-old, respectively. Older and elderly patients (≥40 years) were more likely to have viral suppression than younger patients (18–39 years) (p<0.001). No significant difference in the latest CD4+ T lymphocyte count in the different age strata was observed, although elderly patients (≥ 50 years) had lower CD4+ T lymphocyte nadir (p<0.02). The number of comorbidities increased with age and the same pattern was observed for the majority of the comorbidities, including diabetes mellitus, dyslipidemia, hypertension, cardiovascular diseases, erectile dysfunction, HCV, renal dysfunction and also for non-AIDS-related cancers (p<0.001). With the survival increase associated to successful antiretroviral therapy and with the increasing new infections among elderly group, the burden associated to the diagnosis and treatment of the non-AIDS related HIV comorbidities will grow. Longitudinal studies on the impact of aging on the HIV/AIDS population are still necessary, especially in resource-limited countries.
Worldwide, life expectancy has been increasing over the last several decades, even in developing countries, leading to a greater number of individuals ≥60 years. There will be approximately 2 billion people ≥60 years in the world by 2025, the majority of whom (80%) will reside in developing countries.1 According to the Brazilian Institute of Geography and Statistics (IBGE), 97% of the Brazilian population at the beginning of the 20th century was younger than 59 years; currently, 15.8 million Brazilians are at least 60 years old, and this number is estimated to increase to more than 40 million by 2030. Overall, life expectancy of the Brazilian population by 2030 is estimated to be 78.3 years, 81.9 years for women.2
The introduction of highly active antiretroviral therapy (HAART) during the 1990s was crucial to reduce HIV-related morbidity and mortality rates turning HIV infection into a chronic condition. Antiretroviral therapy (ART) global coverage has significantly grown in the latest years, with 11.7 million life-years added to the world between 1996 and 2008.3 Consequently, the HIV/AIDS population is becoming older. The World Health Organization (WHO) and the majority of geriatricians define “elderly” as a person aged ≥60 years. However, the American Centers for Disease Control and Prevention (CDC) considers a patient with HIV/AIDS at the age of 50 years to be elderly due to the impact of HIV and ART on aging.4 According to the Brazilian Ministry of Health, among the 608,230 cases of HIV/AIDS notified up to June 2011; 64,560 (10.6%) were in the elderly group (≥50 years).5Chronic conditions associated with aging such as non-AIDS-defining malignancies, cardiovascular diseases and other end-organ diseases and deaths attributable to these conditions have also increased in HIV/AIDS cohorts.6–9
The aim of this study was to describe the immunological, clinical and comorbidity profile across age of an urban cohort of patients with HIV/AIDS followed up at a referral center for HIV care and research in Rio de Janeiro, Brazil, in 2008.
MethodsDescription of the clinical cohort and study populationThis study was conducted at Instituto de Pesquisa Clinica Evandro Chagas, Oswaldo Cruz Foundation (IPEC/FIOCRUZ), where care has been provided to HIV/AIDS patients since 1986. An observational, longitudinal, clinical database has been maintained on patients receiving primary HIV care in the clinic. Data are updated regularly using outpatient and inpatient clinical documentation, laboratory testing results, and pharmacy records. Trained abstractors record all this information onto standardized forms for processing. Details of the methodology have been previously described.10
For this cross sectional study, we included retrospective data from all patients over 18 years of age who have had at least one follow-up appointment (clinical visit, lab exams, antiretrovirals (ARV) reload, social assistance interview) between January 1st, 2008 and December 31st, 2008 (n=2307). This study was approved by IPEC/FIOCRUZ ethics committee.
Study definitionsAge in 2008 was the variable of interest across all analyses. Patients were stratified as 18–39 years (“younger patients”), 40–49 years (“older patients”); 50–59 years and ≥60 years (“elderly patients”), according to CDC criteria.4 Other variables used to describe our cohort included demographic, clinical and treatment related characteristics.
For “years since HIV-infection”, we considered the period between the first HIV positive test available and 31 December 2008.
“Viral suppression” was defined as viral load less than 400 HIV RNA copies/μL at all available viral load assessments performed during 2008. For “Last CD4 cells/μL” the last CD4+ T lymphocyte count available in 2008 was considered, while for “Nadir CD4 cells/μL” all historical CD4 results available until 31 December 2008 were considered.
For “Era of starting antiretroviral therapy (ART)”, we classified patients who started using mono/dual (one or two ARV only) or HAART. To classify “ART-naïve” and “current HAART regimen” only year 2008 information was considered. We have also accessed the number of patients on use of new ARV (etravirine, enfurvitide, raltegravir and darunavir).
For “Comorbidities” (diabetes mellitus, dyslipidemia, hypertension, depression and erectile dysfunction) we considered the cases under specific drug intervention during the year 2008. Data were collected from medical prescriptions and medical charts. For “cardiovascular diseases” other than hypertension, reports of cardiac arrhythmia, heart failure, coronary disease, ischemic heart disease, peripheral vascular insufficiency and stroke were considered. For Hepatitis B (HBV), we considered all patients with a positive HBV surface antigen (HBsAg) exam. For Hepatitis C (HCV), we considered all patients with an anti-HCV positive test.
“Renal dysfunction” was defined as a glomerular filtration rate (GFR) lower than 60mL/min calculated by the CKD-EPI for all patients who had a creatinine result in 2008 (n=1971; 85.4%). Patients with hospitalization history within six-month interval of the creatinine result were excluded to avoid bias with acute renal disease (n=37; 1.6%).
For “AIDS defining illness”, “AIDS-defining cancer” and “non-AIDS-defining cancer” all diagnoses until 2008 were considered. We used the Centers for Disease Control (CDC, 1993) for “AIDS defining illness”.
Statistical analysisStatistical comparison of quantitative variables across decade of life was accessed by the Cuzick's non-parametric test for trend. For categorical variables, the Cochran–Armitage non-parametric test for trend was used. For all tests, the threshold for statistical significance was set at 5%. Analyses were done using R-software (version 2.14.2).
ResultsDemographic dataSelected demographic values distributed according to the decade of life are summarized in Table 1. A total of 2307 patients were included in this analysis. From these, 1023 (44.3%) were 18–39 years, 823 (35.7%) were 40–49 years, 352 (15.3%) were 50–59 years, and 109 (4.7%) were ≥60 years. Each age stratum had more male (overall average of 63.6%) and white (overall average of 57.2%) patients.
Demographics, HIV treatment and clinical status of HIV/AIDS patients from IPEC/FIOCRUZ cohort, Rio de Janeiro, Brazil, stratified by age in 2008.
| Variable | 18–39 | 40–49 | 50–59 | ≥60 | Total | p-Value |
|---|---|---|---|---|---|---|
| (n=1023) | (n=823) | (n=352) | (n=109) | (n=2307) | ||
| Sex: Men, n (%) | 631 (61.7) | 542 (65.9) | 228 (64.8) | 67 (61.5) | 1468 (63.6) | 0.335 |
| HIV transmission group: MSMa | 331 (60.7) | 301 (63.6) | 101 (49.3) | 33 (53.2) | 766 (59.6) | 0.021 |
| Race: Non-white | 473 (46.4) | 334 (40.6) | 134 (38.1) | 44 (40.4) | 985 (42.8) | 0.004 |
| Education: <4 years, nr. (%) | 264 (25.8) | 171 (20.8) | 93 (26.6) | 42 (38.5) | 570 (24.7) | 0.150 |
| ART clinical trial enrolment, nr. (%) | 447 (44.3) | 347 (43.1) | 148 (43.1) | 35 (33.6) | 977 (43.2) | 0.129 |
| Age at HIV diagnosis, years | <0.001 | |||||
| Mean (SD) | 27.3 (5.9) | 35.9 (6.1) | 44.2 (6.5) | 55.1 (7.1) | 34.3 (9.8) | |
| Median (IQR) | 27.4 (23.7–31.4) | 36.0 (31.5–40.6) | 44.0 (39.4–49.1) | 54.3 (50.4–59.7) | 33.1 (27.3–40.2) | |
| Years since HIV-infection | <0.001 | |||||
| Mean (SD) | 5.2 (4.4) | 9.1 (5.7) | 10.1 (5.8) | 10.9 (5.6) | 7.6 (5.6) | |
| Median (IQR) | 3.5 (1.9–8.0) | 8.8 (3.8–13.5) | 10.2 (4.9–15.3) | 11.5 (7.1–15.4) | 6.9 (2.6–11.7) | |
| Viral suppression, nr. (%) | 472 (50.4) | 458 (61.6) | 194 (60.6) | 59 (57.3) | 1183 (56.2) | <0.001 |
| Last CD4 cells/μL | 0.490 | |||||
| Mean (SD) | 477 (378) | 491 (299) | 590 (1372) | 497 (309) | 501 (620) | |
| Median (IQR) | 425 (298–605) | 437 (299–624) | 464 (287–657) | 460 (279–690) | 436 (294–622) | |
| Nadir CD4 cells/μL | <0.020 | |||||
| Mean (SD) | 243 (205) | 197 (181) | 191 (168) | 188 (148) | 217 (190) | |
| Median (IQR) | 220 (84–333) | 163 (76–298) | 160 (61–279) | 169 (77–272) | 184 (73–302) | |
| ART-naïve, nr. (%) | 251 (24.5) | 93 (11.3) | 32 (9.1) | 5 (4.6) | 381 (16.5) | <0.001 |
| Years on ART | <0.001 | |||||
| Mean (SD) | 4.6 (4.1) | 7.4 (4.7) | 8.5 (4.9) | 8.8 (5.0) | 6.5 (4.8) | |
| Median (IQR) | 3.0 (1.1–7.7) | 7.8 (2.7–11.5) | 9.5 (4.2–12.4) | 9.8 (3.9–12.5) | 6.4 (1.8–10.8) | |
| Age at ART start, years, median (IQR) | 28.5 (25.0–32.6) | 37.2 (33.3–41.7) | 45.4 (41.2–49.7) | 56.7 (51.8–60.8) | 35.4 (29.5–42.4) | <0.001 |
| Era of starting ART, nr. (%) | ||||||
| HAART | 599 (78.2) | 468 (65.1) | 189 (59.2) | 55 (53.4) | 1311 (68.7) | <0.001 |
| Mono/Dual | 167 (21.8) | 251 (34.9) | 130 (40.7) | 48 (46.6) | 596 (31.2) | |
| Current HAART Regimen, nr. (%) | ||||||
| NNRTI | 400 (51.8) | 299 (41.0) | 122 (38.1) | 32 (30.8) | 853 (44.3) | 0.097 |
| PI | 330 (42.7) | 374 (51.2) | 180 (56.2) | 62 (59.6) | 946 (49.1) | |
| Others | 42 (5.4) | 57 (7.8) | 18 (5.6) | 10 (9.6) | 127 (6.6) | |
| Use of new ARV, nr. (%) | ||||||
| Etravirine | 10 (1.0) | 28 (3.4) | 8 (2.3) | 5 (4.6) | 51 (2.2) | 0.003 |
| Enfurvitide | 9 (0.9) | 28 (3.4) | 14 (4.0) | 3 (2.7) | 54 (2.3) | 0.001 |
| Raltegravir | 15 (1.5) | 19 (2.3) | 10 (2.8) | 4 (3.7) | 48 (2.1) | 0.036 |
| Darunavir | 34 (3.3) | 75 (9.1) | 28 (7.9) | 12 (11.0) | 149 (6.5) | <0.001 |
SD, standard deviation; IQR, interquartile range.
Missing data: race (5); years of education (3); ART clinical trial enrolment (45); viral suppression (203); era of starting ART (19).
The proportion of MSM and of non-whites significantly fluctuated over the age groups (p=0.021 and p<0.001, respectively). MSM Transmission Group was less frequent among elderly (49.3% for 50–59 years; 53.2% for ≥60 years).
There were no statistically significant differences in years of education and enrollment in an ART clinical trial among the age groups.
Treatment and clinical statusHIV treatment and clinical status distributed according to the decades of life are depicted in Table 1. The median age at HIV diagnosis and years since HIV infection significant increased (p<0.001) with age. Consequently, other variables related to ART use (e.g. number of years on ART, use of mono/dual therapy, use of PI-based regimen, use of new ARV) also increased with age, while the proportion of ARV-naïve patients significantly decreased with age (p<0.001).
We found a higher proportion of viral suppression among older and elderly patients (p<0.001). There was no significant difference in the latest CD4+ T lymphocyte count in the different age strata. However, older and elderly patients had lower CD4+ T lymphocyte nadir (p<0.020).
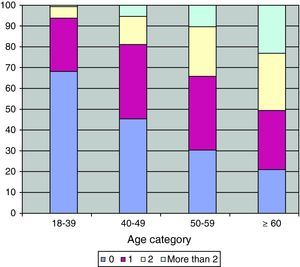
Comorbidities, smoking status and other characteristicsThe number of comorbidities increased with age (Fig. 1). The same pattern was observed for the majority of the comorbidities, including diabetes mellitus, dyslipidemia, hypertension, cardiovascular diseases, erectile dysfunction, HCV, renal dysfunction and also for non-AIDS-related cancers (p<0.001) (Table 2).
Distribution of smoking status, comorbidities, polipharmacy and other characteristics of HIV/AIDS patients from IPEC/FIOCRUZ cohort, Rio de Janeiro, Brazil, stratified by age in 2008.
| Variable | 18–39 (n=1023) | 40–49 (n=823) | 50–59 (n=352) | ≥ 60 (n=109) | Total (n=2307) | p-Value |
|---|---|---|---|---|---|---|
| Smoking status, nr. (%) | ||||||
| Current | 226 (27.8) | 187 (27.4) | 86 (28.5) | 8 (9.8) | 507 (27.0) | 0.067 |
| Quit | 137 (16.8) | 209 (30.6) | 125 (41.4) | 33 (40.2) | 504 (26.8) | <0.001 |
| Never | 450 (55.3) | 285 (41.8) | 90 (29.8) | 40 (48.8) | 865 (46.0) | <0.001 |
| Comorbidities and polipharmacy, nr. (%) | ||||||
| Diabetes mellitus | 2 (0.2) | 14 (1.7) | 15 (4.3) | 5 (4.6) | 36 (1.6) | <0.001 |
| Dislipidemia | 49 (4.8) | 139 (16.9) | 81 (23.0) | 32 (29.4) | 301 (13.0) | <0.001 |
| Hypertension | 57 (5.6) | 109 (13.2) | 76 (21.6) | 33 (30.3) | 275 (11.9) | <0.001 |
| Cardiovascular diseases | 42 (4.1) | 73 (8.8) | 56 (15.9) | 30 (27.5) | 201 (8.7) | <0.001 |
| Estimated glomerular filtration rate (CKD-EPI<60mL/min) | 13 (1.5) | 26 (3.6) | 20 (6.6) | 20 (20.8) | 79 (3.9) | <0.001 |
| Median CKD-EPI, mL/min (IQR) | 120.1 (108.7–133.7) | 107.8 (95.0–120.2) | 100.1 (85.2–111.0) | 92.0 (67.5–102.0) | 111.3 (97.4–124.5) | <0.001 |
| Hepatitis B | 29 (2.9) | 25 (3.1) | 9 (2.6) | 2 (1.9) | 65 (2.9) | 0.580 |
| Hepatitis C | 26 (2.6) | 53 (6.6) | 42 (12.1) | 13 (12.1) | 134 (6.0) | <0.001 |
| Depression | 146 (14.3) | 155 (18.8) | 82 (23.3) | 21 (19.3) | 404 (17.5) | <0.001 |
| Use of anxiolitics, nr. (%) | 154 (15.0) | 171 (20.8) | 84 (23.9) | 16 (14.7) | 425 (18.4) | 0.005 |
| Non-AIDS-related cancers | 6 (0.6) | 15 (1.8) | 8 (2.3) | 6 (5.5) | 35 (1.5) | <0.001 |
| Erectile dysfunctiona | 11 (1.8) | 32 (5.8) | 19 (8.3) | 7 (10.4) | 69 (4.7) | <0.001 |
| Use of female hormones, nr. (%)b | 71 (18.2) | 32 (11.5) | 18 (14.6) | 4 (9.5) | 125 (15.0) | 0.053 |
| Other characteristics, nr. (%) | ||||||
| Menopause, nr. (%)b | 4 (1.0) | 63 (22.7) | 95 (79.2) | 42 (100) | 204 (24.5) | <0.001 |
| AIDS-related cancers, nr. (%) | 21 (2.0) | 38 (4.6) | 12 (3.4) | 3 (2.7) | 74 (3.2) | 0.115 |
| AIDS-defining illness, nr. (%) | 456 (44.6) | 478 (58.1) | 197 (56.0) | 61 (56.0) | 1192 (51.7) | <0.001 |
| Death, nr. (%) | 15 (1.5) | 13 (1.6) | 5 (1.4) | 5 (4.6) | 38 (1.6%) | 0.158 |
SD, standard deviation; IQR, interquartile range.
Missing data: cardiovascular diseases (8); estimated glomerular filtration rate (305), smoking status (427), Hepatitis B (67), Hepatitis C (70).
Depression was observed in 14.3%, 18.8%, 23.3% and 19.3% of individuals aged 18–39 years, 40–49 years, 50–59 years and ≥60 years, respectively (p<0.001). Use of anxiolytics was observed in 15.0%, 20.8%, 23.9% and 14.7% for the groups aged 18–39 years, 40–49 years, 50–59 years and ≥60 years, respectively (p=0.005). For AIDS-defining illnesses, there was a higher proportion of these complications among older and elderly patients (58.1% and 56.0%, respectively) as compared to younger patients (44.6%; p<0.001). There was no significant difference in the proportion of patients with HBV and AIDS-related cancers among the different age groups.
Overall, 27% (507 individuals) were current smokers. There was no significant difference on current smoking status by age strata. However, only 9.8% of patients aged ≥60 years were current smokers, whereas values for patients aged 18–39 years, 40–49 years and 50–59 years were 27.8%, 27.4% and 28.5%, respectively. A higher proportion of elderly patients had quit smoking (p<0.001), and a greater proportion of patients who never smoked were found in the younger (55.3%) and ≥60 years (48.8%) age strata (p<0.001).
Among women in different age strata we found no differences in hormone use (either for contraception or replacement therapy). Menopause was observed in 1.0%, 22.7%, 79.2% and 100% of individuals aged 18–39 years, 40–49 years, 50–59 years and ≥60 years, respectively (p<0.001). Overall, 24.5% of women had undergone menopause.
DiscussionOur results show that there are important variations in several aspects related to HIV infection across the life decades.
Demographic and clinical parameters varied with age in our cohort. Individuals considered elderly (≥50 years) comprised 20% of our cohort, which is low when compared to cohorts from resource rich countries. In the Swiss cohort, for instance, 26% of individuals were 50–64 years old, and 5% were ≥65 years old.7 We found a men to women HIV infection ratio of 1.7, which is similar to the ratio observed in the overall HIV population in Brazil and Rio de Janeiro State,5 but lower than cohorts from developed countries.9,11
Whites were more prevalent in all age groups. Nevertheless, the difference between whites and non-whites was higher among older and elderly individuals. This is in accordance with the trends of the Brazilian epidemic, if we assume race as a proxy for social strata.5
Elderly men (≥50 years) reported less MSM exposure as HIV risk category when compared to younger and older men. The same results have been observed in other studies in Spain and the USA.11,12 This may be related to the fact that elderly individuals may not be comfortable with reporting MSM sexual practices. It has been reported that men at this age are more prone to having sex with multiple partners or to not using condoms,13 therefore behavioral interventions to promote condom use and other prevention strategies should be evaluated in this population.
Elderly individuals had a longer duration of HIV infection when compared with younger patients (10.1 years and 10.9 years for patients aged 50–59 years and ≥60 years, respectively). These values were lower than in the Swiss cohort (15.7 years for ages 50–64 years and 18.2 years for ages ≥65 years).7 Elderly patients (≥50 years) in our cohort were infected younger and are aging, whereas new HIV infections in older individuals are less frequent, especially among those patients aged ≥60 years. These findings suggest that our study population actually is aging with HIV.
Last CD4+ T lymphocyte counts median was slightly higher among elderly patients, although this difference was not statistically significant. This finding could be attributed to a survival bias in older patients with higher CD4+ T lymphocyte counts, or could merely be a reflection of HIV viral replication control after HAART introduction.14 As observed in other cohorts, we found a higher proportion of older and elderly patients who achieved virological responses with HAART when compared with younger patients,7,11,15 and this could be attributed to better adherence to ART.16 In a study conducted in the US, elderly patients were more likely to achieve superior virological responses within one year of HAART, but adjustment for adherence attenuated this finding.17 Unfortunately, there was no structured adherence evaluations for our patient population.
Late presentation in HIV infection is common. Diagnosis is usually done when the immunological system is already compromised and median values of CD4+ T lymphocyte counts are lower than 200cels/mm3.18 Elderly individuals are frequently diagnosed even later than younger patients.19,20 and this is reflected in the lower CD4+ T lymphocyte nadir that we observed among elderly patients in our cohort. Other studies in this population have also observed low CD4+ T lymphocyte nadir21 as well as worse clinical outcomes including a shorter time between HIV identification, AIDS diagnosis, and survival time.15 This may be related to the lower level of suspicion of HIV diagnosis among older adults.19
Because older patients have longer durations of HIV infection, it was expected that this population would be on ART for a longer period of time. According to the Brazilian guidelines, NNRTI-based HAART should be the preferred first-line regimen, and PI regimens as second-line and salvage regimens.22 This could explain the higher prevalence of younger individuals on NNRTI while among older and elderly patients PI-based regimens were more widely used. This was also observed for the use of new drugs (enfurvitide, raltegravir, etravirine and darunavir), although their overall use is still low among all age groups. This could be attributed to the fact that these new drugs can only be used as salvage regimens according to Brazilian ART guidelines.22
The prevalence of current smokers in our cohort was higher than for the Brazilian general population (27.0% vs. 15.5%).23 The prevalence of smoking is usually higher in HIV-infected patients than in the HIV-negative population. Patients aged ≥60 years had the lowest prevalence of current smoking (9.76%), probably due to the fact that many of those older past smokers have already quit smoking. The same profile has been observed in other cohorts from resource rich countries.7,14
Consistent with the literature, 74.3% of our patients ≥60 years and 61.4% of patients aged 50–59 had at least one comorbidity.24,25 In the Brazilian general population, 65% of individuals aged 50–64 years and 79.1% aged ≥65 years have at least one chronic disease.26 Results from a recent study conducted in Italy showed that the prevalence of two or more comorbidities in HIV patients aged 40 years was similar to a control group of HIV-negative patients aged 55 years, which may be translated as premature aging by 15 years.27
The prevalence of diabetes mellitus in Brazilians aged ≥18 years is 5.2%,2 and the current estimated prevalence in the world population is 4.1%.28 These rates are greater than the overall diabetes prevalence (1.6%) in our cohort but similar to the rate observed in the elderly group. The prevalence of diabetes mellitus among HIV-infected patients on ART in a cohort from the US (median age of 48 years) has been reported to be more than 4-fold higher than among seronegative control groups.29 It is important to highlight that our study was mostly comprised by a larger proportion of younger people. Moreover, we have used a strict definition for diabetes in our study, as we only considered as having diabetes those patients who were using specific treatment.
As expected, an association between age and dyslipidemia was observed in our study, with a prevalence of 23% for those patients aged 50–59 years, similar to the observed for patients on lipid-lowering agents aged 50–65 years in the Swiss Cohort.7
Hypertension was detected in 11.9% of all patients in our study, which is similar to the prevalence of hypertension controlled by antihypertensive agents in the Brazilian general population (14.8%).30 Comparing the age groups, the prevalence was 30.3% for patients aged ≥60 years and 21.6% for patients aged 50–59 years whereas it was 5.6% and 13.2% among those patients aged 18–39 and 40–49, respectively. These data are comparable to a HIV/AIDS cohort from the US.31
Patients with HIV/AIDS have a higher risk of cardiovascular diseases (CVD) in the long-term.8 In our cohort, there was a higher prevalence of CVD among elderly patients (15.9% and 27.5%, for 50–59 and ≥60 years, respectively) compared with younger patients, which is higher than the rates observed for the Brazilian general population (8.5% and 15.9%, for 50–64 and ≥65 years, respectively)32 and higher than HIV/AIDS cohorts from Spain (13%)11 and Italy (16%).27 It is important to note that the prevalence of tobacco use in our cohort is high, increasing the risk of CVD. Assessment of risk factors followed by targeted interventions for risk reduction are critical steps for CVD prevention in this patient population.
Glomerular filtration rates (GFR) usually decrease with age, and individuals over 60 years have 20–30% lower GFR than those younger than 50 years.33 The prevalence of GFR <60mL/min in our population was 3.9%, higher than the rate observed in the UK CHIC cohort (2.0%).34 As expected, there was a significant difference in the proportion of reduced GFR among patients ≥60 years (20.8%) and 50–59 years (6.6%) when compared to younger patients (1.5%; p<0.0001), consistent with what was observed in other cohorts.9,11 Earlier ART initiation, renal toxicity monitoring and appropriate comorbidity management are key interventions to preserve a heathy renal function among the HIV/AIDS patients who are getting older.
The prevalence of depression in our cohort was higher than the worldwide prevalence (17.5% vs. 4.10%) but lower than that observed in other HIV cohort studies of other countries, which varied from 20% to 79% depending on the population studied, the duration of the study and the parameters used to define depression.35,36 This may be related to our restrictive definition of depression including only those patients on antidepressants. Patients with milder depression may have been excluded. We observed a high proportion of depression among the older and elderly. A similar profile was observed in the Swiss cohort, although in other cohorts the prevalence of depression was higher only among patients aged ≥60 years.37 The same pattern was observed for the use of anxiolytics (BZD); 18% of our population regularly used one BZD, which is similar to the observed in a cross-sectional survey of 2932 HIV-infected patients in France (16%)38 and much higher than the observed rate in the overall Brazilian population (5.6%).39 This finding highlights the additional burden related to HIV infection and its impact on the quality of life of people living with HIV/AIDS.40
In various studies, when compared to the general population, elderly patients with HIV/AIDS had a relative risk of 1.3 for all non-AIDS-related cancers, especially those related to chronic infections (HPV-anal cancer, Epstein Barr-Hodgkin's disease, and Hepatitis B and C-liver cancer).41,42 Cancer prevalence in our study was higher among patients aged ≥60 years compared with patients aged 50–59 years (5.5% vs. 2.3%) but lower than that observed in the literature for the elderly (13%).11 The most frequently diagnosed cancers in our cohort were skin (37.5%), rectum/anus (18.7%), breast and uterus (9.4% each), lung and stomach (6.2% each), larynx, intestine, prostate and kidney (3.1% each). In the USA HIV/AIDS population, approximately 50% of the estimated non-AIDS-related cancers (n=9645) were cases of lung cancer, anal cancer, liver cancer, and Hodgkin lymphoma, which are known to occur more frequently among people with HIV.43 In the Swiss cohort, the most frequent cancers were lung (10.3%), prostate (7.1%), and skin (5.6%).7 The fact that Brazil is a tropical country and our Institution is located in a beach town could explain the high proportion of skin cancers, which are probably related to non-protected solar exposure. Anal cancer is one of the most common cancers affecting individuals infected with HIV. In a study involving 13 cohorts from North America, anal cancer rates were substantially higher for HIV-infected MSM, other men, and women compared with HIV-uninfected individuals.44
Prevalence of erectile dysfunction (ED) among male patients aged ≥60 and 50–59 years (10.5% and 8.3%, respectively) could have been underestimated due to the absence of free ED drugs available in our hospital, patients failing to report ED drug use, or even the lack of information on ED reported by health professionals. However, these results are in agreement with a previous study from São Paulo (Brazil) with general population, where 12.3% of patients aged ≥60 years and 6.7% of patients aged 50–59 years reported ED.45 In a MSM cohort in California, it was verified that HIV-infected MSM with advanced disease had significantly increased odds for ED when compared to HIV-negative men while HIV-infected MSM with no evidence of AIDS appeared to have a slightly (but statistically nonsignificant) increase in odds for ED.46 These prevalences are higher than those found in our study, probably because they were based on self-reported questionnaires, not restricted to assess the use of ED medications.
We have observed a high prevalence of menopause among women between the ages of 40 and 49 years (22.7%), in accordance with data reported by the Women's Interagency HIV Study that found that median age for menopause was 47.7 years-old.47 In the general population, the median age at menopause is 51 years.48 HIV-infected women tend to reach menopause younger, on average at the age of 46 years.49 De Pommerol and colleagues observed that the average age was 49 years and identified an association with earlier menopause in women with more advanced immunosuppression, defined as lymphocyte T CD4+ counts <200cells/mm3,50
It is important to highlight that the lifestyle of people living with HIV can be different within the HIV context itself and also when compared to the general population. Moreover, the impact of the traditional risk factors may also differ both among distinct groups of people living with HIV, as in the general population. These differences may affect the frequency and the outcomes of the different comorbidities. Finally, these differences can be crucial to define better strategies for the prevention, early identification and treatment of the comorbidities.
In summary, we have observed a higher prevalence of non-AIDS comorbidities, particularly cardiovascular disease, dyslipidemia, renal disease, depression and non-AIDS-defining malignancies among elderly patients. Our data also suggest that menopause may occur earlier among HIV infected women, as observed in other cohorts. Of note, due to the cross-sectional design of our study, causal associations cannot be inferred. Moreover, the retrospective nature of the work, and the lack of more detailed information in medical charts/database on some specific aspects of medication use are potential causes of underestimation of some events such as ED and depression.
With the survival increase associated to successful ART, coupled with the higher rates of new HIV infections among this age group, the burden associated with the diagnosis and treatment of non-AIDS related HIV comorbidities will grow substantially. These results point out the importance of a comprehensive approach in the clinical management of the HIV/AIDS population, including risk factors reduction and preventive screening procedures. Longitudinal studies on the impact of aging on the HIV/AIDS population are warranted, especially in resource-limited countries.
Conflict of interestAll authors declare to have no conflict of interest.